Mercury-Free Future: A Comprehensive Guide to Alternative Electrodes for Stripping Analysis in Biomedical Research
This article provides a critical evaluation of mercury electrode alternatives for stripping analysis, tailored for researchers and professionals in drug development and clinical analysis.
Mercury-Free Future: A Comprehensive Guide to Alternative Electrodes for Stripping Analysis in Biomedical Research
Abstract
This article provides a critical evaluation of mercury electrode alternatives for stripping analysis, tailored for researchers and professionals in drug development and clinical analysis. With increasing regulatory pressure and a global shift towards green chemistry, the move away from traditional mercury electrodes is imperative. We explore the foundational principles, operational methodologies, and practical applications of leading mercury-free electrodes, including bismuth, gold, and carbon-based materials. The content offers a direct performance comparison, troubleshooting guidance for complex matrices like biological fluids, and validation protocols to ensure data reliability. This guide serves as an essential resource for laboratories transitioning to safer, sustainable, and highly sensitive electroanalytical techniques.
Why Move Away from Mercury? The Drive for Safer, Sustainable Electroanalysis
The Toxicity and Environmental Impact of Mercury Electrodes
Mercury electrodes have long been a cornerstone of electrochemical analysis, particularly in stripping voltammetry techniques prized for their exceptional sensitivity in detecting trace metals. Their widespread use, however, belies significant concerns regarding toxicity and environmental impact. This guide objectively examines the performance of traditional mercury-based electrodes against emerging mercury-free alternatives, providing researchers with the data necessary to make informed, responsible choices. The evaluation is framed within a critical thesis: while mercury electrodes remain a performance benchmark, technological advances have rendered mercury-free alternatives not only viable but often preferable when balancing analytical performance with environmental and safety considerations. The discussion encompasses the full lifecycle of these electrodes, from their role in the laboratory to their contribution to the global mercury pollution cycle, offering a comprehensive comparison grounded in experimental data.
Toxicity and Environmental Persistence of Mercury
The utility of mercury electrodes is inextricably linked to the element's intrinsic toxicity and its dangerous pathway through the environment. Mercury is ranked as the third most toxic substance by the US Government Agency for Toxic Substances and Disease Registry, behind only arsenic and lead [1]. Its release into the environment, whether from laboratory waste or industrial processes, initiates a persistent and damaging cycle.
Molecular Mechanisms of Toxicity
The toxicity of mercury manifests at the cellular level through several distinct mechanisms, each contributing to its profound health impacts:
- Binding to Sulfhydryl Groups: Mercury exhibits a high affinity for sulfhydryl (-SH) and thiol groups in proteins and enzymes, leading to macromolecular structural changes and functional disruption [1] [2]. This binding can alter membrane permeability and inactivate critical enzymes.
- Oxidative Stress and Mitochondrial Dysfunction: Mercury exposure induces oxidative stress by promoting the generation of reactive oxygen species (ROS), partly due to its ability to act as a catalyst for Fenton-type reactions [1]. This oxidative damage is coupled with mitochondrial dysfunction, which disrupts cellular energy production and calcium homeostasis [1].
- DNA Damage: Mercury compounds can cause direct damage to DNA, posing a genotoxic risk and potentially contributing to carcinogenesis [1] [2].
Health Impacts and Pharmacokinetics
The health effects of mercury are systemic, affecting nearly every organ system. The nervous system is the primary repository for mercury, where it can accumulate with a half-life as long as 20 years in the brain, leading to neurotoxicity, tremors, sleep disturbances, and impaired cognitive skills [1] [2]. Other documented effects include:
- Cardiovascular and Hematological Effects: Mercury accumulation in the heart is linked to cardiomyopathy, and it can compete with iron for binding sites on hemoglobin, leading to anemia [1].
- Renal and Pulmonary Toxicity: The kidneys are particularly vulnerable, with exposure linked to acute tubular necrosis and glomerulonephritis. Inhalation of mercury vapors can cause pulmonary fibrosis and bronchitis [1] [2].
- Immunological and Reproductive Damage: Mercury impairs immune system function, particularly polymorphonuclear leukocytes (PMNs), increasing susceptibility to infections. It also poses significant risks to the reproductive system and embryonic development [1] [2].
The toxicokinetic profile varies by form. Inhaled elemental mercury vapor is readily absorbed and has a whole-body half-life of approximately 60 days [1]. The most significant human exposure route to organic methylmercury (MeHg) is through consumption of contaminated fish and seafood [1] [2]. Once ingested, MeHg is efficiently absorbed and can bioaccumulate in the food chain, with a biological half-life of 39 to 70 days [2].
The Mercury Lifecycle and Global Impact
The environmental impact of mercury is a global challenge. Human activities have increased global atmospheric mercury levels by three to five times since industrialization [3]. A critical development is the shifting geographical pattern of emissions. While the Global North and China have seen declining emissions in recent decades due to environmental regulations, these reductions have been completely offset by rapid growth in Global South countries [3]. Consequently, global emissions continue to rise slightly since the 2013 Minamata Convention, a global treaty designed to protect human health and the environment from mercury emissions [3].
Table: Global Anthropogenic Mercury Emission Trends (1960-2021)
| Region/Parameter | 1960 Status | 2021 Status | Key Drivers of Change |
|---|---|---|---|
| Global North | Accounted for nearly half of global emissions | Share decreased to only 10% | Strict air pollution controls, shift to renewable energy [3] |
| China | Not a major emitter | Reached 566 Mg in 2021, but shows recent downward trend | Rapid industrial expansion, followed by recent emission controls [3] |
| Global South (excl. China) | Minor emitter | Produced two-thirds of global emissions in 2021 | ASGM, fossil fuel combustion, cement production [3] |
| Largest Emission Sector | Non-ferrous metal production | Artisanal and Small-scale Gold Mining (ASGM) | ASGM emissions hit 975 Mg in 2021, a ten-fold increase from 1960 [3] |
This persistent release ensures mercury continues to circulate in the atmosphere-soil-water distribution cycles, where it can remain for years [1]. The element's volatility allows it to travel long distances, contaminating regions far from the original source. In aquatic systems, mercury is converted by microorganisms into methylmercury, its most toxic form, which then bioaccumulates and biomagnifies in the food web, ultimately reaching humans [2]. This global biogeochemical cycle, exacerbated by ongoing emissions, means that every gram of mercury used in a laboratory setting contributes to a significant and persistent environmental health problem.
Performance Comparison: Mercury vs. Alternative Electrodes
While the toxicity of mercury is clear, its historical use in electroanalysis is rooted in superior electrochemical properties. This section provides a performance-based comparison, evaluating mercury against leading alternatives across key analytical parameters to determine if modern substitutes can meet the demanding requirements of trace metal analysis.
The Legacy Performance of Mercury Electrodes
Mercury electrodes, particularly the Hanging Mercury Drop Electrode (HMDE) and Mercury Film Electrodes (MFE), are considered the gold standard for a reason [4] [5]. Their performance benefits are multifaceted:
- Excellent Conductivity: Mercury provides a highly conductive surface for efficient electron transfer during deposition and stripping steps [5].
- Formation of Amalgams: Mercury's ability to form amalgams with many analytes of interest (e.g., Zn, Cd, Pb, Cu) allows for efficient pre-concentration and well-defined, easily stripped signals [4] [5].
- Renewable Surface: The HMDE offers a perfectly renewable, smooth surface, which is crucial for achieving high reproducibility between measurements [4].
- High Hydrogen Overpotential: This property suppresses hydrogen gas evolution, allowing for the application of very negative deposition potentials necessary to reduce a wider range of metal ions without interference from the solvent breakdown [5].
These properties collectively enable mercury electrodes to achieve remarkable sensitivities, with detection limits capable of reaching 10â»Â¹â° to 10â»Â¹Â¹ M for many metal ions, making them competitive with sophisticated techniques like ICP-MS [4] [5]. MFEs, where a thin mercury film is plated onto a glassy carbon electrode, offer enhanced sensitivity over HMDEs due to their higher surface-to-volume ratio [5].
Promising Mercury-Free Electrode Materials
Significant research efforts have been dedicated to finding less toxic alternatives that match or, in some contexts, surpass mercury's capabilities. The most successful alternatives include:
- Bismuth (Bi): Bismuth is widely regarded as the most promising mercury substitute. It can be used in a similar fashion to form in-situ or ex-situ films and shares key properties with mercury, such as the ability to form alloys with other metals and a relatively high hydrogen overpotential [6] [5]. A key advantage is its compatibility with alkaline media, where mercury forms insoluble oxides, thus expanding the analytical window [5].
- Gold (Au): Gold electrodes are particularly valuable for analyzing metals like mercury itself, as well as arsenic and selenium. They form well-defined intermetallic compounds and exhibit excellent conductivity [4] [5]. A notable application is the
scTRACE Goldelectrode, which has been successfully used to monitor bismuth and antimony(III) stabilizers in electroless nickel plating baths with excellent recovery rates [6]. - Other Materials: Metals like tin (Sn) have also been investigated as film electrodes, though they are less commonly used than bismuth or gold [5]. Composite electrodes, such as graphite electrodes modified with a mercury salt-containing polymer (e.g.,
ItalSens IS-HM), represent a compromise, minimizing the amount of mercury used while attempting to retain its beneficial properties [4].
Table: Comparative Analytical Performance of Electrode Materials in Stripping Voltammetry
| Electrode Material | Key Analytical Strength | Reported Limit of Detection (Example) | Notable Advantages / Disadvantages |
|---|---|---|---|
| Mercury Film Electrode (MFE) | Wide range of amalgam-forming metals | Pb, Cu in biodiesel: Low nM range [5] | Gold standard sensitivity; High toxicity [5] |
| Bismuth Film Electrode (BiFE) | Pb, Cd, Zn, Tl | Pb²âº: 1.4 nM [5] | Low toxicity, works in alkaline media [5] |
| Gold Electrode | Hg, As, Se, Bi, Sb(III) | Effective for process control in plating baths [6] | Highly selective for specific metals; Low toxicity [6] [5] |
| Mercury/Bismuth Composite | Attempts to balance performance and safety | -- | Minimizes mercury use; still contains mercury [4] |
Critical Performance Trade-offs
The transition to mercury-free electrodes involves navigating specific trade-offs. Bismuth electrodes, while excellent for many applications, may not match the extreme negative potential window of mercury in all media, potentially limiting the range of analyzable metals in some cases [5]. Gold electrodes can be susceptible to surface fouling and may require more careful maintenance [5]. Furthermore, the well-established understanding of intermetallic compound formation and its effects in mercury systems is still being developed for these newer materials. However, the experimental data clearly demonstrates that for a core set of environmentally and clinically relevant heavy metals—including Pb, Cd, and Zn—bismuth-based electrodes provide sensitivity and limits of detection that are comparable to their mercury counterparts [5]. This, combined with their lower toxicity and the ability to operate in alkaline conditions, makes them a compelling alternative for most routine analyses.
Experimental Protocols and Research Toolkit
The practical implementation of stripping analysis, whether with mercury or alternative electrodes, requires standardized protocols and a specific set of research reagents. This section details a core methodology and outlines the essential toolkit for conducting these sensitive measurements.
Generalized Stripping Voltammetry Workflow
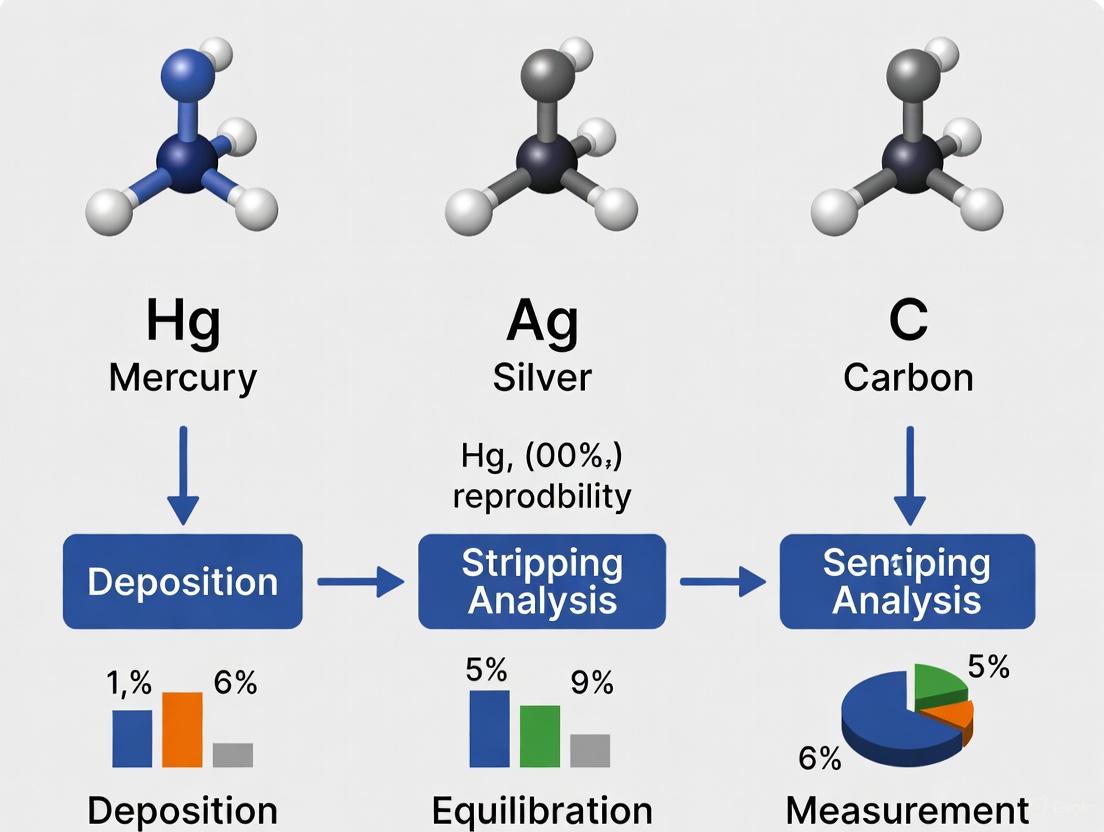
The fundamental steps of a stripping analysis are consistent across electrode types, involving a pre-concentration (deposition) step followed by a measurement (stripping) step. The following diagram and protocol outline a standard anodic stripping voltammetry (ASV) procedure, adaptable for various electrode materials.
Diagram Title: Stripping Voltammetry Workflow
Step-by-Step Protocol:
- Electrode Preparation (Cleaning/Activation): The working electrode must be in a clean, reproducible state. For a glassy carbon electrode, this involves polishing with alumina slurry. For certain mercury-free electrodes like the Bi Drop or
scTRACE Gold, a specific electrochemical activation might be required [6] [5]. - Film Deposition (For in-situ electrodes): For in-situ MFEs or BiFEs, a potential is applied to co-deposit the electrode material (e.g., Hg²⺠or Bi³âº) and the analyte metals onto the inert substrate (e.g., glassy carbon) from the sample solution. This is typically done for 30-300 seconds with stirring [4] [5].
- Analyte Pre-concentration: A cathodic potential (
E_deposition) sufficient to reduce the target metal ions is applied for a fixed time (t_deposition), typically 30-300 seconds, while the solution is stirred. This deposits the metals onto/into the film electrode [4]. - Equilibration: The stirring is stopped, and the solution is allowed to become quiescent for a short period (e.g., 30 seconds). This reduces the capacitive current before the stripping step [4].
- Stripping: A voltammetric technique (e.g., Linear Sweep, Differential Pulse, or Square Wave Voltammetry) is applied to sweep the potential to more anodic values. This oxidizes (strips) the deposited metals back into solution, generating a current peak for each metal [4] [5]. Square Wave Voltammetry is often favored for its sensitivity and speed.
- Data Processing: The resulting voltammogram is analyzed. The potential of each peak identifies the metal, while the peak current or charge is proportional to its concentration in the sample, often determined using a standard addition method [4].
The Researcher's Toolkit
Conducting robust stripping analysis requires a set of specific reagents and instrumentation.
Table: Essential Research Reagents and Equipment for Stripping Analysis
| Item | Function / Description | Example / Note |
|---|---|---|
| Potentiostat | Instrument that applies potential and measures current. | Core of the electrochemical setup. |
| Working Electrode | Surface where deposition and stripping occur. | HMDE, MFE, or Hg-free (e.g., Bi Drop, scTRACE Gold) [6] [4]. |
| Reference Electrode | Provides a stable, known reference potential (e.g., Ag/AgCl). | Essential for accurate potential control. |
| Counter/Auxiliary Electrode | Completes the electrical circuit (e.g., Pt wire). | -- |
| Supporting Electrolyte | Conducting salt solution that minimizes ohmic drop. | e.g., Acetate buffer, HCl, Nitric acid [6] [5]. |
| Standard Solutions | Known concentrations of target metals for calibration. | Used for standard addition method. |
| Complexing Agents | Used in AdSV to form adsorbable complexes with the analyte. | e.g., cupferron, catechol [7]. |
| Purified Gases | Nitrogen or Argon for deaeration to remove dissolved oxygen. | Oxygen can interfere by being reduced. |
| Fosifidancitinib | Fosifidancitinib, CAS:1237168-58-9, MF:C21H21FN5O7P, MW:505.4 g/mol | Chemical Reagent |
| Candicidin A3 | Candicidin A3, CAS:58591-23-4, MF:C59H86N2O18, MW:1111.3 g/mol | Chemical Reagent |
The evidence demonstrates a clear trajectory in electrochemical analysis: the scientific community is moving decisively toward mercury-free alternatives. The severe toxicity and persistent environmental impact of mercury, compounded by its complex global lifecycle and rising emissions in developing nations, render its continued use unsustainable and ethically questionable [1] [3] [2].
While mercury electrodes historically set the standard for sensitivity in stripping voltammetry, the performance gap has narrowed remarkably. Materials like bismuth now offer comparable analytical performance for a wide range of metals, with the added benefits of lower toxicity, compliance with stringent international regulations (e.g., RoHS), and operational flexibility in media like alkaline solutions where mercury fails [6] [5]. Gold and other specialized electrodes further expand the toolbox for specific applications.
For the modern researcher, the choice is no longer between performance and safety. Mercury-free sensors such as the Bi Drop and scTRACE Gold provide a viable, high-performance pathway that aligns with the principles of green chemistry and occupational health. The experimental protocols and data presented herein offer a foundation for laboratories to confidently transition away from mercury, contributing to a reduction in its environmental burden while maintaining the highest standards of analytical rigor. The future of trace metal analysis is unequivocally mercury-free.
The Restriction of Hazardous Substances (RoHS) directive, a pioneering piece of European Union legislation, has fundamentally reshaped the manufacturing of electrical and electronic equipment (EEE) by restricting the use of specific hazardous substances [8]. While often associated with consumer goods, its influence extends powerfully into research laboratories, driving a significant shift away from traditional materials, particularly those containing lead and mercury [9]. The directive's core objective is to reduce environmental and health risks associated with the manufacture, use, and disposal of electronic equipment, promoting the production of safer, more environmentally friendly products [8].
For researchers and scientists, understanding RoHS is no longer merely a matter of regulatory compliance but a crucial component of sustainable and responsible science. The directive restricts ten substances, including lead (0.1% by weight) and mercury (0.1% by weight), with stringent maximum concentration limits in homogeneous materials [8]. Although some research and development equipment may qualify for exemptions, these are often limited in time and scope [8]. The regulatory push is clear, compelling the scientific community to seek out and validate high-performance alternatives to legacy tools, such as mercury-based electrodes in electrochemical stripping analysis, without compromising data quality or analytical performance.
RoHS and Its Direct Impact on Laboratory Instrumentation
The RoHS directive's scope is broad, encompassing most electrical and electronic equipment, which includes a vast array of standard laboratory instruments [8]. For the scientific community, the transition is particularly impactful for analytical techniques that have historically relied on mercury and lead. The directive's substance restrictions are not static; they are subject to ongoing review and tightening, with exemptions for specific applications having finite lifetimes [10] [11].
A key area of focus is the phase-out of mercury. RoHS 3 has introduced time-limited exemptions for mercury in various lamps, including UV lamps used in scientific instrumentation, with many set to expire on February 24, 2027 [11]. Beyond lighting, the pressure is on to replace mercury in other components, such as sensor fill materials, with manufacturers now offering mercury-free and alternative fill sensors specifically for sensitive applications in food and medical sectors [12]. Similarly, the use of lead, particularly in specialized solders for high-reliability equipment like servers and network infrastructure, is under constant review, with exemptions potentially expiring as soon as 2026 [10]. This regulatory environment creates a tangible and urgent need for laboratories to future-proof their analytical methodologies by adopting robust, RoHS-compliant technologies.
Mercury Electrodes in Stripping Analysis: The Traditional Gold Standard
Anodic Stripping Voltammetry (ASV) is a powerful trace electroanalytical technique known for its exceptional sensitivity, with detection limits capable of reaching (sub)nanomolar concentrations [13]. For decades, the Hanging Mercury Drop Electrode (HMDE) has been the quintessential working electrode for this method, and it remains the benchmark against which alternatives are measured [13] [14].
The HMDE's superior performance is attributed to several unique properties [13]:
- Excellent Reproducibility: The electrode surface is easily renewed with each new, identical mercury drop, avoiding hysteresis effects.
- Wide Cathodic Potential Window: Mercury favors a high overpotential for hydrogen evolution, allowing the detection of metals that would otherwise be masked by solvent electrolysis.
- Efficient Amalgam Formation: Many metal ions are efficiently reduced and preconcentrated into the mercury drop, forming amalgams that lead to sharp, well-defined stripping peaks.
These properties have made HMDE the recommended approach in reference laboratories for the simultaneous determination of trace metal ions like Zn²âº, Cd²âº, Pb²âº, and Cu²âº, even in complex matrices like digested soil samples [13]. However, the high toxicity of mercury and its impending regulatory restrictions under directives like RoHS have motivated an intensive search for definitive replacements [13].
Objective Comparison of Mercury Electrode Alternatives
The search for alternatives has yielded several promising electrode materials. The following table provides a structured, quantitative comparison of the HMDE against the most prominent RoHS-compliant candidates, based on reported experimental data.
Table 1: Performance Comparison of Working Electrodes for Stripping Analysis of Metal Ions
| Electrode Type | Key Features | Detection Limit (Hg²âº) | Linear Range (Hg²âº) | Reproducibility (RSD) | Multi-Ion Analysis Capability |
|---|---|---|---|---|---|
| Hanging Mercury Drop Electrode (HMDE) | Renewable surface, forms amalgams, wide potential window [13]. | Not specified for Hg²âº; (sub)nanomolar for other metals [13]. | Not specified for Hg²âº. | Excellent (<5%) [15]. | Excellent for Zn²âº, Cd²âº, Pb²âº, Cu²⺠[13]. |
| Bismuth Film Electrode (BiFE) | RoHS-compliant, "mercury-like" behavior, low toxicity [15]. | Not specified. | Not specified. | Similar to mercury films [15]. | Good for several heavy metals. |
| Nitrogen-Doped Graphene (NRGO) | High affinity for Hg²âº, high surface area, excellent conductivity [16]. | 0.35 nM (0.07 ppb) [16]. | 1 nM - 10 μM [16]. | ~3.8% (for 6 successive measurements) [16]. | Possible, but demonstrated for Hg²âº. |
| Gold Nanoparticle/ Graphene | High sensitivity, catalytic properties [16]. | 6 ppt (0.03 nM) [16]. | Not specified. | Not specified. | Not specified. |
| Sulfur-Doped Porous rGO | Soft-soft interaction with Hg²⺠for high selectivity [16]. | 0.5 nM [16]. | Not specified. | Not specified. | Not specified. |
The data reveals that while HMDE offers unparalleled versatility for multi-ion analysis, advanced nanomaterials like nitrogen-doped graphene can match or even surpass its sensitivity for specific analytes like mercury, achieving detection limits far below the WHO guideline of 30 nM (6 ppb) for drinking water [16]. Bismuth film electrodes present a more direct, lower-tech replacement with performance characteristics closest to traditional mercury films [15].
Detailed Experimental Protocols for Key Alternatives
To facilitate the adoption of these alternatives, detailed and reproducible experimental protocols are essential. Below are methodologies for two prominent RoHS-compliant approaches.
Method 1: Mercury Detection using a Nitrogen-Doped Reduced Graphene Oxide (NRGO) Electrode
This protocol outlines the modification of a glassy carbon electrode (GCE) with NRGO for the highly sensitive detection of mercury ions (Hg²âº), as detailed in the research of Li et al. (2020) [16].
1. Electrode Modification:
- Synthesis of NRGO: Graphene oxide (GO) is thermally treated under an NH₃ atmosphere at 800°C to incorporate nitrogen atoms into the graphene lattice [16].
- Preparation of NRGO Ink: Disperse 2 mg of the synthesized NRGO in 1 mL of a water-ethanol mixture (1:1 v/v) and sonicate for 30 minutes to form a homogeneous suspension [16].
- Modification of GCE: Polish a bare glassy carbon electrode (3 mm diameter) with alumina slurry, rinse thoroughly with deionized water, and dry. Pipette 6 μL of the NRGO ink onto the GCE surface and allow it to dry under an infrared lamp to obtain the NRGO/GCE [16].
2. Experimental Procedure and Parameters:
- Supporting Electrolyte: 0.1 M acetate buffer solution (pH 5.0) [16].
- Pre-concentration/Deposition Step: Immerse the NRGO/GCE in the sample solution containing Hg²âº. Stir the solution at 400 rpm and apply a deposition potential of -0.6 V (vs. Ag/AgCl) for 180 seconds. This step reduces Hg²⺠to Hgâ° and accumulates it on the electrode surface via chelation with the nitrogen sites [16].
- Equilibration: After deposition, stop stirring and allow the solution to become quiescent for 10 seconds [16].
- Stripping Step: Initiate a square-wave anodic stripping voltammetry (SWASV) scan from -0.6 V to +0.4 V. Use the following parameters: frequency of 50 Hz, pulse amplitude of 25 mV, and a step potential of 4 mV [16].
- Regeneration: Between measurements, regenerate the electrode surface by applying a potential of +0.6 V for 60 seconds in a clean supporting electrolyte to oxidize and remove any residual mercury [16].
Method 2: Multi-Metal Analysis using a Bismuth Film Electrode (BiFE)
This protocol is adapted from standard practices for bismuth film electrodes, which are widely recognized as the most practical mercury-free alternative for multi-metal analysis [15].
1. Electrode Preparation:
- Substrate Preparation: Polish a glassy carbon electrode (GCE) or a screen-printed carbon electrode sequentially with 1.0, 0.3, and 0.05 μm alumina slurry. Rinse thoroughly with deionized water between each polishing step [15].
- In-situ Bismuth Film Plating: Introduce the supporting electrolyte (e.g., 0.1 M acetate buffer, pH 4.5) containing a known concentration of Bi(III) ions (typically 200-400 μg/L) and the target analytes. The bismuth film is electroplated onto the substrate in-situ simultaneously with the target metals during the deposition step [15].
2. Experimental Procedure and Parameters:
- Pre-concentration/Deposition Step: Deposit the target metals and bismuth at a potential of -1.4 V (vs. Ag/AgCl) for 60-300 seconds with solution stirring [15].
- Equilibration: Stop stirring and allow a rest period of 10-15 seconds [15].
- Stripping Step: Record the stripping signal using a square-wave voltammetry scan from -1.4 V to +0.2 V. The bismuth and target metals are oxidized (stripped) from the electrode surface at their characteristic potentials, producing distinct peaks for simultaneous multi-metal analysis [15].
The following workflow diagram visualizes the key steps common to stripping analysis, highlighting the points of differentiation between traditional and modern approaches.
Diagram 1: Generalized Workflow for Anodic Stripping Voltammetry. The "Electrode Modification" and "Regeneration" steps are where RoHS-compliant alternatives most significantly diverge from traditional mercury-based methods.
The Scientist's Toolkit: Essential Reagents and Materials
Transitioning to RoHS-compliant stripping analysis requires specific materials. The following table lists key reagents and their functions for the protocols described above.
Table 2: Essential Research Reagents for RoHS-Compliant Stripping Analysis
| Material/Reagent | Function in the Experiment | Exemplary Use-Case |
|---|---|---|
| Glassy Carbon Electrode (GCE) | Provides a pristine, inert, and conductive substrate for electrode modification [16]. | Base electrode for coating with NRGO or for in-situ BiFE plating. |
| Nitrogen-Doped Reduced Graphene Oxide (NRGO) | Electrode modifier; nitrogen atoms act as chelation sites for enhanced pre-concentration of metal ions like Hg²⺠[16]. | Sensitive and selective detection of mercury ions. |
| Bismuth(III) Nitrate | Source of Bi³⺠ions for the in-situ formation of a bismuth film electrode (BiFE) on the carbon substrate [15]. | Multi-metal analysis of Zn²âº, Cd²âº, Pb²âº, etc. |
| Acetate Buffer (pH 4.5-5.0) | Serves as the supporting electrolyte; maintains optimal pH for the deposition and stripping of many heavy metal ions [16]. | Standard electrolyte for ASV of most heavy metals. |
| Standard Metal Ion Solutions | Used for calibration curves to quantify the concentration of unknown analytes in samples. | Essential for all quantitative analysis. |
| Fructose-arginine | Fructose-arginine, CAS:25020-14-8, MF:C12H24N4O7, MW:336.34 g/mol | Chemical Reagent |
| Fulacimstat | Fulacimstat, CAS:1488354-15-9, MF:C23H16F3N3O6, MW:487.4 g/mol | Chemical Reagent |
The regulatory drive embodied by RoHS is unequivocally accelerating innovation in electrochemical research. While the hanging mercury drop electrode has set a high bar for analytical performance, the scientific community has responded with sophisticated alternatives that are not only compliant but also highly competitive. Materials like nitrogen-doped graphene demonstrate that it is possible to achieve exceptional, single-analyte sensitivity surpassing even traditional methods [16]. Meanwhile, more established options like bismuth film electrodes offer a robust and practical path for routine multi-analyte trace metal detection [15].
The transition to a lead- and mercury-free lab is no longer a hypothetical future but a present-day reality. By understanding the regulatory landscape, objectively evaluating the performance of new technologies against traditional benchmarks, and adopting standardized experimental protocols, researchers can confidently navigate this shift. The move toward RoHS-compliant methodologies represents a convergence of regulatory necessity and scientific progress, fostering the development of analytical chemistry that is not only more sustainable and safe but also more advanced.
Stripping analysis is a powerful electrochemical technique renowned for its exceptional sensitivity in trace metal detection. Its core principle hinges on a two-step process that separates the signal generation in time from the analyte collection, enabling the detection of metal ions at concentrations as low as the nanomolar and sub-nanomolar level [17] [13]. For decades, the hanging mercury drop electrode (HMDE) was the cornerstone of this technique due to its excellent reproducibility, wide cathodic potential window, and ability to form amalgams with many metals [13]. However, owing to the high toxicity of mercury, the field has vigorously pursued "green" alternative electrode materials, primarily bismuth (Bi), but also antimony (Sb), tin (Sn), and gold (Au) [18] [19].
The following diagram illustrates the foundational two-step workflow of anodic stripping voltammetry, which is common to both traditional and modern electrodes.
The Two-Step Mechanism for Ultra-Sensitive Detection
The remarkable sensitivity of stripping analysis is achieved by de-coupling the detection event from a preliminary analyte preconcentration phase [18] [13].
- Preconcentration and Equilibration: In this first step, the target metal ions (e.g., Cd²âº, Pb²âº, Zn²âº) in solution are electrochemically reduced and deposited onto the working electrode surface. A constant potential is applied, and the solution is stirred, leading to the accumulation of the metal as a thin film or, in the case of mercury, an amalgam [13]. This step concentrates the analytes from a large sample volume onto a small electrode surface, effectively amplifying the future signal. A quiet, unstirred equilibration period often follows to ensure a uniform concentration profile at the electrode surface [13].
- Stripping and Quantification: The second step is the actual measurement. The potential is scanned in an anodic (positive) direction, causing the accumulated metals to oxidize back into ions and re-dissolve into the solution [13]. This "stripping" process generates a measurable current. Each metal oxidizes at a characteristic potential, producing distinct peaks in the voltammogram. The height or area of these peaks is directly proportional to the concentration of the metal in the original sample, allowing for precise quantification [17] [19].
Quantitative Comparison of Electrode Performance
The search for viable mercury alternatives has yielded several promising materials. The table below summarizes the key analytical performance metrics of these electrodes for the detection of common heavy metals, based on experimental data from the literature.
Table 1: Performance Comparison of Electrodes for Stripping Analysis of Heavy Metals
| Electrode Type | Target Metals | Linear Range (mol/L or µg/mL) | Limit of Detection (LOD) | Key Advantages | Key Limitations | Experimental Context |
|---|---|---|---|---|---|---|
| Hanging Mercury Drop Electrode (HMDE) [13] | Zn²âº, Cd²âº, Pb²âº, Cu²⺠| Not specified | (Sub)nanomolar | Excellent reproducibility; Wide potential window; Ideal for multi-ion analysis [13]. | High toxicity of mercury [13]. | Analysis of digested soil samples [13]. |
| Bismuth-Film Electrode (BiFE) on Copper [17] | Cd²âº, Pb²âº, Zn²⺠| 2x10â»â¸ to 1x10â»â¶ mol/L (for Cd²âº) | Not specified | "Environmentally friendly"; Low toxicity; Well-defined peaks with low background [17]. | Requires acidic media (pH < 4.3); Bismuth hydroxide forms at higher pH [17]. | Analysis in acidified tap water and plant extracts [17]. |
| Bismuth-Film on Paper-Based Carbon [19] | Cd(II), Pb(II), In(III) | 0.1 to 10 µg/mL | 0.4 µg/mL (Cd), 0.1 µg/mL (Pb) | Sustainable, low-cost, and easily disposable platform [19]. | Less sensitive than mercury films; Could not determine Cu(II) [19]. | Determination of metals in tap water [19]. |
| Mercury-Film on Paper-Based Carbon [19] | Cd(II), Pb(II), In(III), Cu(II) | 0.1 to 10 µg/mL | 0.04 µg/mL (In), 0.1 µg/mL (Pb), 0.2 µg/mL (Cu) | High sensitivity; capable of detecting a wider range of metals [19]. | Higher toxicity and associated handling/disposal concerns [19]. | Direct comparison with bismuth films on the same platform [19]. |
| Gold-Plated/ Nanoparticle SPE [18] | Hg, Pb, As, Cu | Varies by application | Excellent for Hg and As | Excellent for Hg and As determination; uses underpotential deposition (UPD) for enhanced sensitivity [18]. | Not a general replacement for all metals; best for specific applications [18]. | Used in automated systems and for Hg determination in urine [18]. |
Detailed Experimental Protocols
To ensure reproducibility, the methodology for electrode preparation and measurement must be precisely defined.
Protocol 1: Ex Situ Preparation of a Bismuth-Film Electrode (BiFE)
This protocol is adapted from studies using carbon-based substrates [19].
- Electrode Preparation: Begin with a clean carbon working electrode (e.g., glassy carbon, screen-printed carbon, or a paper-based carbon electrode).
- Film Deposition: Place the electrode in a separate plating solution containing a bismuth salt (e.g., 10â»Â³ M Bi(III) in a 0.1 M acetate buffer with 0.5 M Naâ‚‚SOâ‚„ as a supporting electrolyte, pH 4.0) [19].
- Electrodeposition: Apply a constant, negative deposition potential (e.g., -1.0 V vs. Ag/AgCl) for a set time (e.g., 60-120 seconds) with solution stirring. This reduces Bi³⺠ions to Biâ°, forming a thin bismuth film on the electrode surface.
- Rinsing: Remove the electrode from the plating solution, rinse it gently with deionized water, and then transfer it to the sample solution for analysis [19].
Protocol 2: Anodic Stripping Voltammetry (ASV) Measurement
This general protocol is used following electrode preparation, whether for HMDE, BiFE, or other film electrodes [13].
- Sample Deaeration: Place the sample solution (e.g., in 0.1 M acetate buffer, pH 4.0) in the electrochemical cell. Purge with an inert gas like nitrogen or argon for approximately 10 minutes to remove dissolved oxygen, which can interfere with the analysis [13].
- Preconcentration (Deposition): Immerse the working electrode. While stirring the solution, apply a constant deposition potential (e.g., -1.1 V for Zn, Cd, and Pb) for a fixed time (e.g., 120 seconds). This causes the reduction and deposition of target metal ions onto the electrode.
- Equilibration: Stop stirring and allow the solution to become quiescent for a short period (e.g., 30 seconds) while maintaining or slightly adjusting the potential [13].
- Stripping Scan: Initiate the voltammetric scan. Using a technique like Differential Pulse Voltammetry (DPV) or Square-Wave Voltammetry (SWV), scan the potential in the anodic direction. In DPV, for example, a pulse amplitude of 25 mV and a pulse step of 4 mV might be used [17] [13]. The oxidation of each metal produces a characteristic current peak.
- Data Analysis: Measure the peak currents. The concentration of the analytes is determined by constructing a calibration curve of peak current versus standard concentration or by using the standard addition method [13].
The interplay between the preconcentration and stripping steps, and how it leads to high sensitivity, is visualized below.
The Researcher's Toolkit: Essential Reagents and Materials
Table 2: Key Research Reagent Solutions for Stripping Analysis
| Item | Function in Stripping Analysis |
|---|---|
| Bismuth(III) Salt (e.g., from a standard ICP solution) [19] | The precursor for forming the bismuth-film working electrode, either ex situ or in situ. |
| Acetate Buffer (pH ~4.0) [19] | A common supporting electrolyte that provides a controlled ionic strength and acidic pH, essential for the deposition and stability of bismuth films. |
| Supporting Electrolyte (e.g., Na₂SO₄, KNO₃) [19] | Carries the current in the solution, minimizes ohmic drop, and defines the ionic medium. |
| Metal Ion Standard Solutions (e.g., Cd²âº, Pb²âº) [17] [19] | Used for calibration curves and the standard addition method to quantify analyte concentrations in unknown samples. |
| Screen-Printed Electrode (SPE) Cards [18] [19] | Disposable, mass-produced electrochemical cells (working, reference, and counter electrodes) that offer convenience and reproducibility. |
| Paper-Based Carbon Electrodes [19] | Ultra-low-cost, hydrophilic, and easily disposable substrates that are particularly suited for decentralized analysis. |
| (R)-Funapide | (R)-Funapide, CAS:1259933-16-8, MF:C22H14F3NO5, MW:429.3 g/mol |
| G007-LK | G007-LK, MF:C25H16ClN7O3S, MW:530.0 g/mol |
In conclusion, while the HMDE remains a benchmark for performance in stripping analysis due to its unparalleled sensitivity and reproducibility [13], bismuth-film electrodes have emerged as a truly viable, environmentally friendly alternative for many applications, especially for the detection of Cd, Pb, and Zn [17] [19]. The choice of electrode involves a trade-off between the superior analytical performance of mercury and the significantly reduced toxicity and practical advantages of bismuth and other "green" metals. The ongoing research in material science, particularly in nanostructuring and new substrate engineering, continues to narrow this performance gap, further solidifying the role of stripping analysis as a critical tool for trace metal detection.
Electrochemical stripping analysis is a powerful trace-level analytical technique renowned for its exceptional sensitivity, with detection limits often reaching the parts-per-trillion (ppt) level [15] [20]. For decades, mercury electrodes were the cornerstone of this method due to their excellent electrochemical properties, including a wide cathodic potential window and renewable surface [21]. However, the high toxicity of mercury and associated legal restrictions on its use and disposal have driven the scientific community to develop effective, environmentally friendly alternatives [22] [23].
This guide objectively compares the performance of the four most prominent "green" electrode materials—Bismuth, Antimony, Gold, and Carbon—that have emerged as viable replacements for mercury in stripping analysis. Framed within the broader thesis of advancing mercury-free electrochemical research, this article provides researchers and scientists with a detailed comparison of these alternatives, supported by experimental data and protocols to inform their selection and application in analytical methods and drug development.
Material Properties and Performance Comparison
The following sections detail the properties of each mercury-free electrode material, and their performance is summarized quantitatively in Table 1.
Bismuth (Bi)
- Overview and Properties: The bismuth-film electrode (BiFE), introduced in 2000, is widely regarded as the most successful mercury alternative [22] [24]. Bismuth is relatively non-toxic and forms "fusing" alloys with heavy metals like lead and cadmium, analogous to mercury amalgams [23]. Its performance is comparable to that of mercury-film electrodes (MFEs), with a wide accessible potential window (typically from -1.2 V to -0.2 V vs. Ag/AgCl) [24].
- Typical Fabrication: BiFEs are commonly prepared by in-situ or ex-situ electroplating of a bismuth salt onto a carbon substrate like glassy carbon or a screen-printed carbon electrode (SPCE) [22] [24]. A key advantage is the ability to perform analysis in non-deaerated solutions, simplifying the experimental procedure [24].
- Electroanalytical Performance: Bismuth electrodes exhibit well-defined, sharp stripping peaks for several trace metals. For example, a bismuth-coated carbon electrode achieved a detection limit of 0.3 µg/L (ppb) for lead following a 10-minute deposition period [24]. The electrodes also show high reproducibility, with relative standard deviations (RSD) of 2.4% and 4.4% for repetitive measurements of Cd and Pb, respectively [24].
Antimony (Sb)
- Overview and Properties: The antimony-film electrode (SbFE), introduced more recently, offers an interesting performance profile with unique electroanalytical characteristics [22] [25]. Like bismuth, antimony has significantly lower toxicity than mercury.
- Typical Fabrication: SbFEs are also prepared by electroplating onto a substrate [25]. Recent research focuses on optimizing the plating conditions and degree of substrate coverage to enhance electrochemical performance, which is critically evaluated using redox probes like Neutral Red [25].
- Electroanalytical Performance: Antimony electrodes are particularly useful for determining metals like nickel(II) and have been successfully applied in complex matrices such as wastewater [25]. They demonstrate a low charge transfer resistance (as low as 6 Ω was reported for a well-covered SbFE), which contributes to their sensitive response [25].
Gold (Au)
- Overview and Properties: Gold electrodes are not a new material but are exceptionally well-suited for specific applications. Their high affinity for mercury and arsenic makes them the best choice for determining these elements [22]. The phenomenon of underpotential deposition (UPD) of metals like Hg and Pb on gold enhances deposition efficiency and sensitivity [22].
- Typical Fabrication: Gold-modified SPCEs can be fabricated by electroplating a thin coat of gold (often forming nanoparticles) or by using commercially available gold-loaded carbon inks [22].
- Electroanalytical Performance: Gold electrodes are predominantly used for detecting Hg and As. A notable application involved a "wearable" sensor on neoprene textile with a gold-plated SPE for determining copper in marine environments, showcasing its potential for field analysis [22].
Carbon-Based Electrodes
- Overview and Properties: Carbon materials, including glassy carbon, carbon paste, and graphite-epoxy composites, serve as the most common substrates for modified electrodes [23]. Their appeal lies in their chemical inertness, wide potential window, and low cost.
- Typical Fabrication: The versatility of carbon is evident in various designs. Screen-printed carbon electrodes (SPCEs) allow for mass production of disposable sensors [22]. Graphite-epoxy composite electrodes (GECE) offer robustness and the advantage of being easily polished to renew their surface [23].
- Electroanalytical Performance: While unmodified carbon electrodes can be used for some analytes, their primary role is as a substrate for other modifier materials like Bi, Sb, or Au. The composite structure can lead to a higher signal-to-noise ratio and improved detection limits [23].
Table 1: Comparative Electroanalytical Performance of Mercury-Free Electrodes for Key Metal Ions
| Electrode Material | Target Analytes | Detection Limit (ppb) | Linear Range | Reproducibility (RSD%) | Key Advantage |
|---|---|---|---|---|---|
| Bismuth (BiFE) | Cd, Pb, Tl, Zn | 0.3 (for Pb) [24] | Low ppb range [24] | 2.4–4.4% [24] | Performance closest to mercury |
| Antimony (SbFE) | Ni, Cd, Pb, Cu | Varies by analyte [25] | -- | -- | Low charge transfer resistance [25] |
| Gold (Au) | Hg, As, Cu, Pb | -- | -- | -- | Best for Hg and As analysis [22] |
| Carbon (GECE) | Cd, Pb, Zn | -- | -- | -- | Robust, inexpensive substrate [23] |
Experimental Protocols for Electrode Preparation and Use
To ensure reproducible and reliable results, standardized experimental protocols are essential. Below is a generalized workflow for anodic stripping voltammetry (ASV), followed by material-specific preparation methods.
Generalized Anodic Stripping Voltammetry Workflow
The core sequence of steps in a typical stripping analysis is consistent across different electrode materials [15] [26]:
- Preconcentration/Deposition Step: A negative potential is applied to the working electrode in a stirred solution, reducing metal ions (Mâ¿âº) and depositing them onto the electrode surface (e.g., as an amalgam in Bi or as a film on Au). The deposition time (30 s to 10 min) and potential are optimized for the target analytes [26].
- Rest Period: Stirring is stopped, and the potential is maintained for a short period (e.g., 5-30 s) to allow the deposited metals to distribute evenly and for the solution to become quiescent [15] [26].
- Stripping Step: The potential is swept anodically (from negative to positive) using a voltammetric technique (e.g., square-wave or differential pulse). The deposited metals are re-oxidized (stripped), generating a characteristic current peak for each metal. The peak current is proportional to the analyte concentration in the original solution [15] [26].
Material-Specific Electrode Preparation Protocols
Protocol for In-Situ Bismuth-Film Electrode (BiFE) [24]:
- Use a glassy carbon or screen-printed carbon electrode as the substrate.
- Prepare a sample or standard solution containing the target metal ions and add Bi(III) to a final concentration of 400 µg/L.
- Simultaneously deposit the bismuth and target metals by applying a deposition potential of -1.3 V (vs. Ag/AgCl) for 2-5 minutes in the stirred solution.
- Proceed with the rest and stripping steps. The bismuth film is formed and stripped simultaneously with the analytes in each cycle.
Protocol for Ex-Situ Antimony-Film Electrode (SbFE) [25]:
- Prepare a separate plating solution containing a salt of Sb(III), such as potassium antimony tartrate.
- Immerse a clean screen-printed carbon electrode (SPCE) into the plating solution.
- Apply a constant potential or current to electrodeposit the antimony film onto the SPCE surface. The specific potentiostatic conditions (potential and time) determine the morphology and coverage of the film, which directly impact performance.
- Remove the modified SbFE, rinse it, and then place it into the sample solution for the stripping analysis.
Protocol for Gold-Modified Electrodes [22]:
- For electroplating, immerse a carbon SPE in a solution containing Au(III) (e.g., from HAuClâ‚„).
- Apply a reducing potential to deposit Au nanoparticles onto the carbon surface.
- Alternatively, use commercially available gold nanoparticle dispersions and drop-cast a small volume onto the electrode surface, allowing it to dry.
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful implementation of stripping analysis with these electrodes requires a set of essential reagents and materials.
Table 2: Key Research Reagent Solutions and Materials
| Item Name | Function / Description | Example Application / Note |
|---|---|---|
| Bismuth(III) Salt | Precursor for forming the bismuth film on the electrode. | E.g., Bismuth citrate; used for in-situ plating of BiFEs [24]. |
| Antimony(III) Salt | Precursor for forming the antimony film on the electrode. | E.g., Potassium antimony tartrate; used for ex-situ plating of SbFEs [25]. |
| Gold Plating Solution | Source of Au(III) ions for electrode modification. | E.g., Tetrachloroauric acid solution; used to electroplate gold nanoparticles onto SPCEs [22]. |
| Supporting Electrolyte | Conducting medium that minimizes resistive losses and controls pH. | E.g., Acetate buffer (0.1 M, pH ~4.5) or HCl (10 mM) [26] [23]. |
| Standard Metal Solutions | Calibrants for quantitative analysis. | Aqueous standards of Cd(II), Pb(II), etc., at mg/L or µg/L concentrations [26]. |
| Screen-Printed Electrodes (SPEs) | Disposable, mass-produced electrochemical cells. | Carbon SPEs serve as a versatile and inexpensive substrate for modification with Bi, Sb, or Au [22]. |
| Complexing Ligand | Enables adsorptive stripping voltammetry (AdSV) for non-electroactive metals. | E.g., Dimethylglyoxime for Ni(II) and Co(II) determination [22]. |
| Galloflavin Potassium | Galloflavin Potassium, MF:C12H5KO8, MW:316.26 g/mol | Chemical Reagent |
| Garvagliptin | Garvagliptin, CAS:1601479-87-1, MF:C18H23F2N3O3S, MW:399.5 g/mol | Chemical Reagent |
Selection Guide and Concluding Outlook
The choice of electrode material is dictated by the specific analytical problem. The following diagram provides a logical pathway for material selection, and the concluding remarks look toward future developments.
The field of mercury-free electroanalysis is dynamic, with current research focused on several promising fronts. The synthesis and application of bimetallic nanomaterials, such as bismuth-antimonate nanosheets, aim to harness synergistic effects to further improve sensitivity and stability [27] [28]. Furthermore, the drive towards decentralized analysis continues to fuel the development of disposable, miniaturized screen-printed sensors modified with these "green" metals for on-site environmental and clinical monitoring [22]. As these materials and fabrication techniques evolve, the performance gap with mercury will continue to narrow, solidifying the role of bismuth, antimony, gold, and carbon as the foundational materials for the future of sustainable stripping analysis.
Implementing Mercury-Free Electrodes: Protocols for Biomedical and Clinical Samples
The accurate detection of trace heavy metals in biological fluids like blood and urine is a critical challenge in clinical, occupational, and environmental health. For decades, anodic stripping voltammetry (ASV) using mercury-based electrodes has been the cornerstone of electrochemical trace metal analysis due to its exceptional sensitivity and reproducibility [19]. However, the high toxicity of mercury has driven the scientific community to seek safer, environmentally friendly alternatives [17] [19]. This pursuit has positioned bismuth-film electrodes (BiFEs) as a leading candidate, combining low toxicity with exemplary electrochemical performance [17] [29]. This guide provides a comparative analysis of bismuth-film electrodes against other mercury-free alternatives, focusing on their preparation, optimization, and application for detecting heavy metals in complex biological matrices such as blood and urine, thereby framing their role within the broader thesis of mercury replacement in stripping analysis research.
Competing Electrode Platforms for Stripping Analysis
The transition away from mercury electrodes has led to the development and refinement of several alternative platforms. The following table offers a structured comparison of their key characteristics.
Table 1: Comparison of Electrode Platforms for Anodic Stripping Voltammetry of Heavy Metals
| Electrode Type | Key Advantages | Key Limitations | Representative Performance (LOD for Pb²âº) | Suitability for Blood/Urine |
|---|---|---|---|---|
| Mercury Film Electrodes (MFEs) | High sensitivity; Excellent reproducibility; Wide negative potential window [19] | High toxicity; Specialized waste disposal [19] | ~0.1 µg/mL [19] | Limited due to toxicity and matrix effects |
| Bismuth Film Electrodes (BiFEs) | Low toxicity; "Environmentally friendly"; High sensitivity comparable to Hg; Well-defined stripping signals [17] [19] [29] | Performance can degrade above pH ~4.3 due to hydroxide formation [17] [30] | Sub-µg/mL to ng/mL range (varies with substrate) [31] [17] | Excellent (demonstrated in urine and blood sera) [31] [30] |
| Graphite-Epoxy Composite Electrodes (GECE) | Mercury-free; Simple design; Robust; Behave as microelectrode array [32] | Generally lower sensitivity compared to BiFEs and MFEs [32] | ~1 ppb (µg/L) [32] | Moderate (requires acidic media) |
| Bismuth Bulk Electrodes (BiBE) | No film deposition required; Effective in neutral pH samples (e.g., urine, rainwater) [30] | Not a thin film; Requires more bismuth material | 8.09 mg/L for Zn(II) (in urine) [30] | Excellent for direct analysis of undisturbed samples [30] |
Experimental Protocols for Bismuth Film Electrodes
Substrate Selection and Preparation
The substrate electrode forms the conductive foundation for the bismuth film. Common choices include:
- Screen-Printed Carbon Electrodes (SPCEs): Ideal for disposable, low-cost sensors. The surface is typically used as-received after verification of conductivity [19].
- Glassy Carbon Electrodes (GCEs): For reusable platforms, the surface must be meticulously polished with alumina slurry (e.g., 0.05 µm) on a microcloth pad, followed by sonication and rinsing with deionized water to create a mirror-finish, reproducible surface [17].
- Paper-Based Carbon Electrodes: A sustainable, low-cost option. These are fabricated by wax-printing hydrophobic barriers on chromatography paper, followed by drop-casting a carbon ink suspension to create the working electrode zone [19] [29].
Bismuth Film Deposition:Ex SituandIn SituMethods
The bismuth film can be formed on the substrate via two primary methods, with ex situ offering greater control for complex matrices.
- Ex Situ Deposition: This involves electroplating the bismuth film onto the substrate in a separate solution prior to sample analysis. A common protocol involves placing the electrode in a deaerated solution of 5-10 mg/L Bi(III) in 0.1 M acetate buffer (pH ~4.0) or 0.1 M HNO₃. A deposition potential of -0.8 V to -1.0 V (vs. Ag/AgCl) is applied for 60-240 seconds with stirring. This pre-plates a uniform, adherent bismuth film [19] [30].
- In Situ Deposition: Here, a Bi(III) salt (e.g., 100-400 µg/L Bi(III)) is added directly to the acidified sample solution. During the preconcentration step, both the target metals and bismuth are simultaneously deposited onto the electrode surface, forming the film in situ. This method is simpler but offers less control over film morphology [17] [19].
Analysis of Heavy Metals in Blood and Urine
The following workflow details the optimized protocol for biological samples, based on studies analyzing Pb²⺠in blood sera and Zn²⺠in urine [31] [30].
- Sample Pre-treatment: Blood sera or urine samples typically require dilution (e.g., 1:1) and acidification with a supporting electrolyte like 0.1 M acetate buffer (pH 4.0) or 0.1 M HNO₃. This step decomplexes metals from proteins and other organic ligands and ensures an optimal pH for deposition [31] [30].
- Preconcentration/Deposition: Transfer the prepared sample to the electrochemical cell. For ex situ BiFEs, apply a deposition potential (e.g., -1.2 V to -1.4 V for Zn, -1.0 V for Pb and Cd) for a controlled time (30-300 s) with stirring. This reduces and accumulates the target metal ions into the bismuth film, forming an amalgam [31] [30].
- Stripping and Measurement: After a brief equilibration period (5-15 s), initiate the stripping step. A square-wave or differential pulse anodic potential scan is applied from the deposition potential to a more positive potential (e.g., -1.0 V to -0.2 V). The metals are re-oxidized ("stripped") from the amalgam, producing characteristic current peaks at specific potentials (e.g., Pb ~ -0.5 V, Cd ~ -0.7 V vs. Ag/AgCl) [31] [17].
- Quantification: The peak current is proportional to the concentration of the metal in the solution. Quantification is typically performed using the standard addition method to compensate for matrix effects in complex samples like blood and urine [19] [30].
Figure 1: Experimental workflow for heavy metal analysis using ex situ bismuth film electrodes.
Performance Data and Comparison
The effectiveness of BiFEs is demonstrated by direct comparisons with mercury films and other alternatives, supported by quantitative experimental data.
Table 2: Experimental Detection Limits and Linear Ranges for Heavy Metals on Different Electrodes
| Electrode | Metal Ion | Linear Range | Detection Limit | Matrix | Source |
|---|---|---|---|---|---|
| BiFEs on HAp-Carbon | Pb²⺠| Not Specified | Clear peaks at -0.55V | Blood Sera | [31] |
| BiFEs on Paper Carbon | Pb²⺠| Up to 10 µg/mL | ~0.1 µg/mL | Tap Water | [19] [29] |
| Mercury Films on Paper Carbon | Pb²⺠| 0.1 - 10 µg/mL | 0.1 µg/mL | Tap Water | [19] [29] |
| Bismuth Bulk Electrode | Zn²⺠| 20 - 160 µg/L | 8.09 µg/L | Urine | [30] |
| Graphite-Epoxy Composite | Pb²⺠| Not Specified | 1 µg/L (ppb) | Standard Solution | [32] |
The data shows that while mercury films may still hold a slight edge in absolute sensitivity for some metals, bismuth-based electrodes provide analytically relevant detection limits that are suitable for monitoring toxic metals like lead and cadmium at clinically and environmentally significant levels.
Optimization and Troubleshooting
- pH Management: The formation of insoluble bismuth hydroxide above pH 4.3 is a major constraint [17]. This makes sample acidification mandatory. The bismuth bulk rotating disk electrode (BiB-RDE) has been successfully used in neutral pH samples like urine, offering a path to analyze undisturbed samples [30].
- Intermetallic Compounds: The formation of intermetallic compounds between co-deposited metals (e.g., Cu-Zn) can distort signals. This can be mitigated by optimizing the deposition potential, adding a chemical masking agent (e.g., gallium), or using a shorter deposition time [30].
- Fouling in Biological Matrices: Proteins and other macromolecules in blood and urine can foul the electrode surface. Sample dilution and acidification are the primary countermeasures. The use of paper-based substrates, which can act as a filter, also helps mitigate this issue [19].
Figure 2: A decision pathway for selecting the appropriate bismuth electrode and deposition method based on sample properties.
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table lists key materials and reagents required for developing and working with bismuth film electrodes.
Table 3: Essential Research Reagents and Materials for Bismuth Film Electrode Research
| Item | Function/Application | Example Specifications |
|---|---|---|
| Bismuth Standard Solution | Source of Bi(III) for film formation | 1000 mg/L Bi(III) in 2-5% HNO₃ (e.g., from Fluka Analytical) [19] |
| Acetate Buffer | Supporting electrolyte; controls pH | 0.1 M, pH ~4.0, with 0.5 M Naâ‚‚SOâ‚„ as background electrolyte [31] [19] |
| Metal Standard Solutions | For calibration and quantification | 1000 mg/L certified standards of Pb, Cd, Zn, Cu in dilute acid [19] |
| Screen-Printed Electrodes (SPCEs) | Disposable electrode substrate | DRP-110 (Carbon working/auxiliary, Ag reference) from Metrohm-Dropsens [19] |
| Glassy Carbon Electrode (GCE) | Reusable electrode substrate | 3 mm diameter, polished with 0.05 µm alumina slurry [17] |
| Wax Printer & Chromatography Paper | For fabricating paper-based electrodes | Xerox ColorQube, Whatman Grade 1 paper [19] |
| Potentiostat with Software | Instrumentation for voltammetric measurements | Autolab PGSTAT with GPES software; equipment capable of SWV and DPV [19] |
| GB1107 | GB1107, MF:C20H16Cl2F3N3O4S, MW:522.3 g/mol | Chemical Reagent |
| Gcn2-IN-1 | Gcn2-IN-1, MF:C19H18N10O, MW:402.4 g/mol | Chemical Reagent |
Bismuth-film electrodes have unequivocally emerged as a viable, environmentally friendly successor to mercury-based electrodes for the stripping voltammetry of heavy metals. Their performance in terms of sensitivity, detection limit, and ability to handle complex biomatrices like blood and urine is comparable, and in some cases superior, to other mercury-free alternatives like graphite-epoxy composites. While challenges such as optimal performance in acidic media persist, ongoing research into electrode substrates and bismuth nanostructures continues to broaden their application scope. For researchers and drug development professionals requiring reliable, sensitive, and sustainable tools for metal detection in biological systems, bismuth-film electrodes represent a mature and compelling technology within the landscape of modern analytical chemistry.
The search for robust alternatives to mercury electrodes represents a significant trend in modern electroanalytical chemistry. For decades, stripping voltammetry has been recognized for its exceptional sensitivity in trace element analysis, yet the toxicity of mercury electrodes has driven the development of solid-state alternatives. Among these, solid gold electrodes have emerged as powerful, environmentally friendly platforms capable of determining numerous toxic elements at regulatory levels. This comparison guide objectively evaluates the performance of the scTRACE Gold electrode—a prominent commercial solid-state sensor—against other gold-based electrodes reported in recent research. The assessment focuses on analytical figures of merit, practical implementation requirements, and applicability for speciation analysis, providing scientists with critical data for selecting appropriate methodologies for their specific trace element determination needs.
Gold electrodes exhibit particular affinity for elements like arsenic and mercury, forming amalgams or intermetallic compounds that enable highly sensitive stripping analysis. The scTRACE Gold electrode specifically addresses several practical limitations of traditional gold electrodes through its unique design featuring an integrated three-electrode system on a single printed platform [33] [34]. This guide examines how such design innovations translate to performance advantages in real-world applications, particularly for researchers monitoring trace metals in environmental, food, and clinical matrices.
Technical Comparison of Gold Electrode Platforms
Design and Operational Characteristics
The scTRACE Gold electrode incorporates a gold micro-wire working electrode thinner than a human hair, with reference and auxiliary electrodes screen-printed on the reverse side [33] [34]. This integrated design eliminates the need for separate electrodes and makes reference electrode maintenance obsolete. A significant practical advantage is its short preparation time—the electrode is ready for use within minutes without extensive preconditioning [33] [35].
In contrast, traditional solid gold electrodes and gold nanoparticle-modified glassy carbon electrodes (AuNPs-GCE) typically require three-electrode cells with separate components. These often involve more complex preparation procedures, including mechanical polishing, electrochemical activation, or nanoparticle deposition steps [36] [37]. Gold electrodes fabricated from recordable CDs (CD-R) represent a low-cost alternative, manufactured by extracting the gold reflective layer from commercial CDs and encapsulating it with epoxy resin [38]. While extremely economical, these homemade electrodes require laborious fabrication and lack standardization.
Modification Strategies for Enhanced Functionality
A key advancement in gold electrode technology is the application of surface modifications to expand analytical capabilities. The scTRACE Gold electrode can be enhanced with thin metallic films to determine elements that show poor response on bare gold:
- Silver film modification enables highly sensitive determination of lead, with detection limits of 0.4 µg/L using the 884 Professional VA system [39] [40].
- Bismuth film modification facilitates the determination of nickel and cobalt as their dimethylglyoxime (DMG) complexes, transferring established methods from mercury electrodes to a mercury-free platform [39].
- Mercury film modification allows determination of chromium(VI) as a complex with diethylenetriaminepentaacetic acid (DTPA), providing detection limits of 2 µg/L despite the intention to avoid mercury [39] [40].
These modification approaches demonstrate how the scTRACE Gold platform can be adapted to specific analytical challenges while maintaining the practical advantages of a solid-state electrode system.
Performance Data Comparison
Table 1: Analytical performance of scTRACE Gold electrode for trace element determination in water
| Analyte | Matrix | Detection Limit (µg/L) | Recovery (%) | RSD (%) | Method Details |
|---|---|---|---|---|---|
| Arsenic | Drinking water | 1.0 | 92 (at 10 µg/L) | 6.5 | Direct determination [33] |
| Copper | Surface water | 0.5 | 107 (at 5 µg/L) | 2.0 | Direct determination [33] |
| Iron | Drinking water | 10 | 91 (at 20 µg/L) | 1.0 | Direct determination [33] |
| Lead | Drinking water | 0.4 (lab), 0.6 (portable) | 96 (at 10 µg/L) | 5.0 | With Ag film modification [39] |
| Nickel | Drinking water | 0.2 (lab), 1.0 (portable) | 99 (at 1 µg/L) | 5.0 | With Bi film modification, as DMG complex [39] |
| Chromium(VI) | Drinking water | 2 | 115 (at 30 µg/L) | 2.0 | With Hg film modification, as DTPA complex [39] |
Table 2: Comparison of gold electrode platforms for mercury determination in biological samples
| Electrode Type | Matrix | Detection Limit | Quantification Limit | Comparison Method | Reference |
|---|---|---|---|---|---|
| scTRACE Gold (unmodified) | Water | ~1 µg/L (inferred) | Not specified | ICP | [33] |
| Solid Gold Electrode (SGE) | Fish | Not specified | <1 mg/kg (wet weight) | DMA, CV-AAS | [36] |
| Au Nanoparticle-Modified GCE | Fish | Not specified | 0.06 mg/kg (wet weight) | DMA | [36] [37] |
| CD-R Gold Electrode | Fish | 0.30 µg/L | 1.0 µg/L | CVAAS | [38] |
Experimental Protocols for Key Applications
Determination of Arsenic in Drinking Water with scTRACE Gold
Methodology Summary: The protocol employs direct anodic stripping voltammetry without electrode modification. After simply connecting the scTRACE Gold electrode to the voltammetric analyzer, the sample is acidified with hydrochloric acid to approximately 0.1 M concentration [33] [34]. The measurement sequence includes a deposition step at a negative potential where arsenic is reduced and deposited onto the gold surface, followed by a stripping scan from negative to positive potentials where the deposited arsenic is oxidized back into solution, producing the analytical signal.
Key Parameters: Deposition potential: -0.4 V; Deposition time: 60-120 s; Stripping technique: Square-wave anodic stripping voltammetry; Analysis time: ~10 minutes per sample [33] [35].
Performance Notes: The method achieves a detection limit of 1 µg/L, one-tenth of the WHO guideline value of 10 µg/L, with a recovery of 92% at the regulatory limit and relative standard deviation of 6.5% [33]. This demonstrates sufficient precision and accuracy for compliance monitoring of arsenic in drinking water.
Mercury Determination in Fish with Gold Nanoparticle-Modified Electrodes
Sample Preparation: Two digestion approaches were compared: conventional microwave digestion using concentrated nitric acid and a simplified field digestion procedure using a commercial food warmer with nitric acid [37]. The field method provides adequate sample preparation for voltammetric analysis while being more accessible for on-site applications.
Electrode Preparation: Gold nanoparticle-modified glassy carbon electrodes (AuNPs-GCE) were prepared by electrodeposition from a solution of tetrachloroauric acid, creating a high-surface-area platform that enhances mercury preconcentration [36] [37].
Analysis Parameters: Square-wave anodic stripping voltammetry (SW-ASV) was performed with a deposition potential of -0.4 V for 300 seconds, followed by a square-wave scan from -0.4 V to +0.4 V. The method was validated against Direct Mercury Analysis (DMA) and Cold Vapor Atomic Absorption Spectroscopy (CV-AAS) [36] [37].
Performance Notes: The AuNPs-GCE achieved a quantification limit of 0.06 mg/kg in fish tissue, performance comparable to DMA, with excellent agreement between laboratory and field preparation methods [37].
Analytical Workflows Diagram
Figure 1: Experimental workflow for trace element analysis using different gold electrode platforms, highlighting key decision points and procedural variations.
Research Reagent Solutions and Essential Materials
Table 3: Essential research reagents and materials for trace element speciation with gold electrodes
| Reagent/Material | Function | Application Examples | Notes |
|---|---|---|---|
| scTRACE Gold electrode | Working electrode with integrated reference/counter | All applications | Ready-to-use, no maintenance required [33] |
| Tetrachloroauric acid | Source for gold nanoparticle electrodeposition | AuNP-modified electrode preparation | Enables creation of high-surface-area electrodes [36] |
| Dimethylglyoxime (DMG) | Complexing agent for nickel and cobalt | Ni/Co determination in water | Forms electroactive complexes for adsorptive stripping [39] |
| Diethylenetriaminepentaacetic acid (DTPA) | Complexing agent for chromium(VI) | Cr(VI) determination in water | Enables speciation of toxic Cr(VI) form [39] |
| Silver nitrate | Source for silver film modification | Lead determination | Renewable surface extends electrode lifetime [39] |
| Bismuth nitrate | Source for bismuth film modification | Nickel/cobalt determination | Mercury-free alternative for these elements [39] |
| Hydrochloric acid (0.05-0.1 M) | Supporting electrolyte and sample acidification | Arsenic, copper determination | Optimal concentration varies by application [33] [38] |
Comparative Advantages and Limitations
scTRACE Gold Electrode System
Advantages:
- Integrated three-electrode design eliminates additional electrode costs and maintenance [33] [34]
- Rapid preparation time enables immediate use after connection [33] [35]
- Modification capability expands determinable elements [39] [40]
- Compatibility with both laboratory (884 Professional VA) and portable (946 Portable VA) instruments facilitates field analysis [33] [39]
Limitations:
- Higher initial cost compared to homemade electrodes
- Limited to predetermined surface area and geometry
- May still require mercury films for certain applications (e.g., Cr(VI)), counteracting mercury-reduction goals [39]
Alternative Gold Electrode Platforms
Solid Gold Electrodes (SGE):
- Provide well-established electrochemistry with extensive literature support [36]
- Require regular polishing and electrochemical pretreatment
- Need separate reference and counter electrodes
Gold Nanoparticle-Modified Electrodes (AuNPs-GCE):
- Enhanced sensitivity due to increased surface area [36] [37]
- Require careful optimization of nanoparticle deposition parameters
- May exhibit better antifouling properties in complex matrices
CD-R-Based Gold Electrodes:
- Extremely low cost (fabricated from commercial CDs) [38]
- Suitable for resource-limited settings
- Lack reproducibility between batches
- Time-consuming fabrication process
The scTRACE Gold electrode represents a significant advancement in solid-state electrode technology, offering practical advantages for routine analysis through its integrated design and simplified operation. While traditional solid gold electrodes and nanoparticle-modified variants continue to provide viable alternatives—particularly for specialized applications or budget-constrained environments—the scTRACE Gold system delivers a balanced combination of performance, practicality, and flexibility for most trace element monitoring scenarios.
For researchers transitioning from mercury-based electrodes, the modification strategies available for the scTRACE Gold platform enable determination of a wide range of elements previously accessible only with mercury electrodes. The analytical performance summarized in this guide demonstrates that modern gold electrodes generally meet or exceed regulatory requirements for trace metal determination in environmental and food matrices, solidifying their position as fundamental tools in contemporary stripping analysis research.
Antimony-Based Electrodes for Monitoring Stabilizers in Compliance with RoHS
The Restriction of Hazardous Substances (RoHS) directive significantly influences material selection in electronic equipment and analytical chemistry by restricting specific dangerous substances [41]. For researchers monitoring trace heavy metals and stabilizers, this has accelerated the search for alternatives to traditional mercury electrodes, which are limited due to mercury's toxicity [17]. Among the most promising "environmentally friendly" alternatives are electrodes based on bismuth and antimony (Sb) [17] [25].
Antimony-based electrodes offer a compelling combination of analytical performance and regulatory alignment. While not currently restricted under RoHS, antimony's use is subject to specific limitations, particularly in children's products and food contact materials, making its compliant application a key research focus [42]. This guide objectively compares the performance of antimony-film electrodes with other prominent alternatives, providing researchers with the experimental data and protocols needed for their application in compliant analysis.
Performance Comparison of Mercury-Alternative Electrodes
The following table summarizes the key performance characteristics of antimony-based electrodes alongside other common mercury-free alternatives, based on recent experimental findings.
Table 1: Performance Comparison of Electrodes for Anodic Stripping Voltammetry
| Electrode Type | Typical Substrate | Sensitivity / Performance | Linear Range (for Cd²âº) | Detection Limit | Key Advantages | Main Limitations |
|---|---|---|---|---|---|---|
| Antimony Film (SbFE) | Screen-Printed Carbon (SPCE) | Low charge transfer resistance (6 Ω); High sensitivity to Ni(II) [25] | Not specified | Not specified | "Green" metal; Low cost; Suitable for novel sensor development [25] | Performance dependent on film coverage [25] |
| Bismuth Film (BiFE) | Glassy Carbon, Copper | Well-defined peaks, low background [17] | 2x10â»â¸ to 1x10â»â¶ mol Lâ»Â¹ [17] | Not specified | "Environmentally friendly"; Low toxicity; High reproducibility [17] | Requires acidic media (pH < 4.3) [17] |
| Tin Film (SnFE) | Not specified | Effective for simultaneous determination of Cr(III) & Cd(II) [25] | Not specified | Not specified | Effective alternative [25] | Not extensively covered in results |
| Mercury Film (HgFE) | Platinum | Historical reference standard | - | - | High reproducibility, sensitivity [17] | High toxicity; Environmental and safety concerns [17] |
Experimental Protocols for Electrode Preparation and Testing
Fabrication of Antimony Film Screen-Printed Carbon Electrodes (Sb/SPCEs)
The performance of SbFEs is highly dependent on the fabrication process, particularly the pre-plating conditions and the resulting coverage of the substrate [25].
- Modification Principle: Antimony is deposited onto a screen-printed carbon electrode (SPCE) surface via a potentiostatic electrodeposition process. The properties of the resulting film can be tuned by varying the deposition potential and time [25].
- Electrode Characterization: The modified electrodes should be characterized using a combination of techniques to correlate structure with function.
- Cyclic Voltammetry (CV) & Electrochemical Impedance Spectroscopy (EIS): Used to evaluate electrochemical performance and charge transfer resistance (Rct). Lower Rct values indicate higher electron transfer rates, a sign of a well-prepared electrode [25].
- Scanning Electron Microscopy (SEM): Provides morphological data on the antimony film, revealing the degree and uniformity of substrate coverage [25].
- Key Finding: Research has demonstrated a strong correlation between the degree of antimony coverage, the electrode's roughness factor, and its resulting sensitivity towards metal ions like nickel (II), confirming that the modification process directly controls the electroanalytical properties [25].
Electroanalytical Measurement of Heavy Metals
- Redox Probe Selection: Neutral Red (NR) has been identified as an effective redox probe for evaluating the electrochemical performance of Sb/SPCEs. In its protonated form, NR undergoes quasi-reversible redox transformations in phosphate buffer solutions (pH 5.5 ± 0.5) within a potential window where antimony itself is not electroactive, providing a clean signal [25].
- Stripping Voltammetry Protocol: The general workflow for determining trace metals involves two main steps [43]:
- Preconcentration: The target metal ions (e.g., Cd²âº, Pb²âº, Ni²âº) are electrochemically reduced and deposited onto the SbFE surface at a controlled potential and time.
- Stripping Measurement: The deposited metals are re-oxidized back into solution using a voltammetric technique like differential pulse voltammetry (DPV). The resulting current peaks are measured, with their position being metal-specific and their intensity proportional to concentration [17] [25].
The following diagram illustrates the sequential workflow for electrode preparation and analysis.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Reagents and Materials for Developing Antimony-Based Sensors
| Item | Function / Application |
|---|---|
| Screen-Printed Carbon Electrodes (SPCEs) | Disposable, economical substrate for constructing portable sensors [25]. |
| Antimony Chloride (SbCl₂/SbCl₃) | Precursor salt for the electrodeposition of the antimony film [25]. |
| Neutral Red (NR) | Redox probe used to evaluate the electrochemical performance and active surface area of the SbFE without interfering with the metal's electroactivity [25]. |
| Phosphate Buffer Solution (pH 5.5±0.5) | Electrolyte medium for characterizing the electrode with Neutral Red and for analyses requiring a near-neutral pH [25]. |
| Nitric Acid / Acetate Buffer | Acidic supporting electrolyte required for the stripping analysis of heavy metals like Cd(II) and Pb(II) to prevent hydrolysis [17]. |
| Metal Ion Standard Solutions | (e.g., Ni²âº, Cd²âº, Pb²âº) Used for calibration and validation of the electroanalytical method [17] [25]. |
| Inavolisib | Inavolisib, CAS:2060571-02-8, MF:C18H19F2N5O4, MW:407.4 g/mol |
| Paxalisib | Paxalisib, CAS:1382979-44-3, MF:C18H22N8O2, MW:382.4 g/mol |
Regulatory Context and Environmental Profile
Understanding the regulatory status of antimony is crucial for its application in RoHS-compliant monitoring.
- RoHS Status: Antimony trioxide (Sb₂O₃) has been reviewed for restriction under the RoHS directive. As of the most recent assessment (December 2019), it was concluded that antimony trioxide should not be restricted under RoHS, though it may be reconsidered in a future review [42]. The International Antimony Association (i2a) maintains that its use in electronic and electrical equipment (EEE) is safe and does not meet the criteria for restriction [42].
- Specific Limitations: Despite the RoHS exemption, antimony is subject to other regulations. For instance, in the EU, the Toy Safety Directive 2009/48/EC sets specific migration limits for antimony from toy materials [42]. Furthermore, its use as a catalyst in PET plastics is regulated with a specific migration limit into food of 0.04 mg/kg [42].
- "Green" Metal Claim: In electrochemical literature, antimony is often categorized alongside bismuth as an "environmentally friendly" alternative to mercury, primarily due to its significantly lower toxicity [25]. This makes it an attractive material for developing new sensors aimed at on-site and environmentally sustainable monitoring.
Antimony-based electrodes present a viable and high-performance alternative to mercury electrodes for the stripping analysis of heavy metals, aligning with the goals of RoHS compliance. Their key advantages reside in their "green" profile, low cost, and excellent electrochemical performance, particularly when optimized for film coverage. While bismuth-film electrodes offer a well-established and highly sensitive platform, their limitation to acidic media can be a constraint. Antimony electrodes thus provide researchers with a crucial tool in the expanding toolkit for compliant and environmentally conscious analytical chemistry. Future developments will likely focus on further improving electrode stability and expanding the application range to a broader spectrum of pollutants.
Mercury-Free Potentiometric Stripping Analysis (PSA) for Zinc and Lead Detection
The determination of trace heavy metals, such as lead and zinc, in environmental and biological samples is crucial for environmental monitoring, industrial process control, and public health protection. For decades, stripping analysis techniques, known for their high sensitivity, have relied heavily on mercury-based electrodes. However, growing environmental and health concerns regarding the toxicity of mercury have spurred significant research into developing robust, mercury-free alternatives. This guide objectively compares the performance of several prominent mercury-free electrodes and methods for the detection of zinc and lead using Potentiometric Stripping Analysis (PSA) and related techniques. The transition to mercury-free electroanalysis represents a critical evolution in green analytical chemistry, aiming to maintain high analytical performance while eliminating hazardous materials.
Performance Comparison of Mercury-Free Electrodes and Methods
The following table summarizes the key performance metrics of various mercury-free electrodes and detection methods for lead and zinc, as reported in the literature.
Table 1: Performance Comparison of Mercury-Free Methods for Lead and Zinc Detection
| Detection Method | Electrode Type | Target Metal | Linear Range | Detection Limit | Key Advantages |
|---|---|---|---|---|---|
| Potentiometric Stripping Analysis (PSA) [44] [45] | Activated Glassy Carbon | Zinc | 0–2000 ppb | Not Specified | Mercury-free electrode and electrolyte; Suitable for trace analysis. |
| Potentiometric Stripping Analysis (PSA) [44] [45] | Freshly Polished Glassy Carbon | Lead | 0–2000 ppb | Not Specified | Mercury-free; Simple electrode preparation (polishing). |
| Potentiometric Stripping Analysis (PSA) [46] | Gold-Coated Screen-Printed | Lead | Not Specified | 0.1 – 0.6 µg/L | Disposable; No oxygen removal; Minimal surfactant interference. |
| Square-Wave Anodic Stripping Voltammetry (SWASV) [47] | Nanoporous Gold (PAA-g-PVDF) | Zinc | 10–1000 µg/L | 4.2 µg/L | Disposable; Effective in oil-polluted seawater; Passive adsorption pre-concentration. |
| Square-Wave Anodic Stripping Voltammetry (SWASV) [48] | Gold Screen-Printed | Lead | Not Specified | 0.5 µg/L | Disposable; Good sensitivity and reproducibility; No plating required. |
| Direct Potentiometry (ISE) [49] | Solid-Contact Ion-Selective Electrode | Lead, Cadmium | Down to nM range | 0.2 nM (Cd); 2.0 nM (Pb) | High selectivity vs. Tl, In, Sn; No preconcentration step. |
The data reveals that both Potentiometric Stripping Analysis (PSA) and Square-Wave Anodic Stripping Voltammetry (SWASV) can be effectively deployed on mercury-free platforms. Glassy carbon, particularly with an activation step for zinc, and various gold-based electrodes (from coated to nanoporous structures) are the most common successful alternatives. These methods consistently achieve detection limits in the microgram per liter (µg/L or ppb) range or lower, which is sufficient for monitoring trace metals in compliance with many environmental regulations, such as the OSPAR standards for production water [47]. The development of disposable screen-printed sensors is particularly notable for on-site, large-scale screening applications, as they offer a practical and environmentally friendly solution by avoiding electrode contamination and enabling mass production [46] [48].
Detailed Experimental Protocols for Key Methods
PSA with Activated Glassy Carbon Electrode for Zinc and Lead
This protocol is adapted from the mercury-free PSA method for the simultaneous analysis of lead and zinc, detailing the specific requirements for electrode preparation and analysis [44] [45].
Electrode Preparation: A glassy carbon working electrode is used in a standard three-electrode cell with a saturated calomel reference electrode (SCE) and a platinum counter electrode.
- For Lead Analysis: The glassy carbon electrode is used after a simple fresh polishing procedure.
- For Zinc Analysis: The glassy carbon electrode requires an activation procedure. This is performed by pre-concentrating zinc in a mercury-free electrolyte containing 0.1 M HCl and 2 ppm Zn²⺠at a deposition potential of -1400 mV (vs. SCE), followed by a stripping step at approximately -1050 mV.
Supporting Electrolyte: A 0.1 M hydrochloric acid (HCl) solution is used.
PSA Measurement:
- Pre-concentration/Deposition: The metal ions (Zn²âº, Pb²âº) are electrochemically reduced and deposited onto the working electrode surface by applying a constant negative potential. For zinc, the activation potential is used.
- Stripping: The oxidation (stripping) of the deposited metals is achieved by disconnecting the applied potential and allowing a chemical oxidant (often dissolved oxygen) to re-oxidize the metals. The process is recorded as potential vs. time.
- Signal Output: The analytical signal is the derivative of the potentiometric stripping curve (dt/dE vs. E), which produces peaks for each metal. The area under these peaks is proportional to the metal concentration in the sample.
Calibration: A linear relationship between the stripping peak area and concentration is established in the range of 0 to 2000 ppb for both lead and zinc.
SWASV with Gold Screen-Printed Sensor for Lead
This protocol outlines the use of disposable, mass-producible gold sensors for the sensitive detection of lead, highlighting a different electrochemical technique and electrode design [48].
Sensor Design: A planar three-electrode strip, comprising a gold working electrode, a carbon counter electrode, and a silver pseudo-reference electrode, all fabricated by screen-printing technology.
Electrode Pretreatment: Prior to analysis, the gold screen-printed electrode is electrochemically pretreated by performing cyclic voltammetry in 0.1 M HCl between -0.7 V and +0.9 V (vs. the Ag pseudo-reference) for several cycles until a stable voltammogram is obtained.
Supporting Electrolyte: 0.1 M HCl.
SWASV Measurement:
- Accumulation/Deposition: A deposition potential (e.g., -0.8 V) is applied to the working electrode for a set time (e.g., 120 seconds) while the solution is stirred. This causes the reduction and deposition of Pb(II) to Pb(0) on the gold surface.
- Stripping: After a brief equilibration period, the square-wave anodic stripping voltammogram is recorded by scanning the potential in a positive direction from the deposition potential. The square-wave modulation enhances sensitivity and speed.
- Signal Output: The analytical signal is an oxidation current peak around -0.5 V (vs. Ag pseudo-reference), corresponding to the re-oxidation of lead. The peak height or area is proportional to the lead concentration.
Performance: This method achieved a detection limit of 0.5 µg/L for lead with a 120-second accumulation time.
Workflow and Selectivity in Mercury-Free Stripping Analysis
The following diagram illustrates the logical progression and decision points involved in selecting and applying a mercury-free stripping analysis method for zinc and lead detection.
A critical aspect highlighted in recent research is the complementary nature of different electrochemical techniques. While stripping voltammetry is renowned for its sensitivity, Potentiometric Stripping Analysis (PSA) and Potentiometry with Ion-Selective Electrodes (ISEs) can offer superior selectivity in certain scenarios. For instance, a direct comparison study found that modern cadmium and lead ISEs exhibited significantly higher selectivity against common interferents like thallium (Tl), indium (In), and tin (Sn), which often complicate stripping voltammetric analysis [49]. This makes PSA and ISEs particularly advantageous for analyzing complex samples where these interferents are present in excess. The choice between these techniques should, therefore, be guided by the specific sample matrix and analytical problem, or they can be used in parallel to provide richer analytical information.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Reagents and Materials for Mercury-Free PSA Research
| Item | Function/Application |
|---|---|
| Glassy Carbon Electrode | A versatile, solid working electrode substrate that can be used polished for lead analysis or activated for zinc detection [44] [45]. |
| Gold Screen-Printed Electrode (AuSPE) | Disposable, mass-produced sensor for decentralized analysis; the gold surface facilitates the deposition and stripping of lead [46] [48]. |
| Nanoporous Gold Electrode | Sputtered on functionalized membranes (e.g., PAA-g-PVDF); provides high surface area for enhanced pre-concentration, useful in complex matrices like oil-polluted water [47]. |
| Hydrochloric Acid (HCl), 0.1 M | A common supporting electrolyte (acidic medium) that provides conductive medium and defines the electrochemical window for deposition and stripping [44] [48]. |
| Acetate Buffer (pH 4.6 - 5.5) | A common buffered supporting electrolyte used in various stripping analyses, including those with bismuth-coated electrodes [49] [47]. |
| Metal Ionophores (e.g., Lead Ionophore IV) | Selective complexing agents embedded in polymer membranes for constructing ion-selective electrodes (ISEs) for direct potentiometry [49]. |
| Bismuth Nitrate | Source for in-situ or ex-situ plating of bismuth films on electrodes, creating a popular "environmentally friendly" replacement for mercury films in anodic stripping voltammetry [49]. |
| GDC-0575 | GDC-0575, MF:C16H20BrN5O, MW:378.27 g/mol |
| GDC0575 hydrochloride | GDC0575 hydrochloride, CAS:1196504-54-7, MF:C16H21BrClN5O, MW:414.73 |
The determination of heavy metals, particularly lead, in biological and environmental samples represents a critical public health priority due to the severe toxicological impacts of these elements [50]. For decades, electrochemical stripping analysis utilizing mercury-based electrodes served as the cornerstone for trace metal detection due to mercury's exceptional electroanalytical performance, including its wide cathodic potential window and reproducible surface renewal [19] [51]. However, growing environmental and safety concerns regarding mercury's toxicity have driven intensive research into alternative electrode materials [19] [50]. Within this context, bismuth-based electrodes have emerged as the most promising "green" alternative, offering comparable performance to mercury without the associated toxicity [51] [50]. This case study examines the application of bismuth film electrodes for the determination of lead in blood samples, positioning this technology within the broader framework of mercury alternative research for stripping analysis.
Bismuth Electrodes: A "Green" Alternative with Comparable Performance
Fundamental Properties and Advantages
Bismuth-based electrodes function similarly to mercury electrodes through the formation of "fused alloys" with heavy metals like lead during the electrochemical accumulation step, analogous to amalgamation at mercury electrodes [50]. This alloying process enables highly sensitive stripping analysis for trace metal detection. Bismuth possesses several inherent advantages that make it particularly suitable for environmental and biological monitoring:
- Low Toxicity: Bismuth is recognized as non-toxic and environmentally friendly, classified among the "green" elements, which eliminates the handling and disposal concerns associated with mercury [51] [50].
- Insensitivity to Dissolved Oxygen: Unlike many electrode materials, bismuth electrodes can function effectively in non-deoxygenated solutions, simplifying analytical procedures by eliminating the need for lengthy nitrogen purging [51] [52].
- Wide Operational Potential Window: Bismuth exhibits a useful negative potential range similar to mercury, allowing detection of metals like lead and cadmium at trace levels [19] [53].
- Low Background Current: The favorable electrocatalytic properties of bismuth result in low background signals, enhancing signal-to-noise ratios and lowering detection limits [51].
Comparative Performance: Bismuth vs. Mercury Electrodes
Extensive research has demonstrated that bismuth film electrodes (BiFEs) can achieve analytical performance comparable to their mercury counterparts for lead detection. The following table summarizes key performance metrics established in validation studies:
Table 1: Performance Comparison of Bismuth and Mercury Electrodes for Lead Detection
| Electrode Type | Detection Limit (μg/L) | Linear Range | Reproducibility (RSD%) | Key Applications Demonstrated |
|---|---|---|---|---|
| Bismuth Film Electrode (BiFE) | 0.05-11.5 [53] [54] | 10-500 μg/L [55] | 1.4-4.3% [52] | Water, biological samples, food [50] |
| Mercury Film Electrode | 0.1 [19] | 0.1-10 μg/mL [19] | Similar to BiFE | Water, environmental samples [19] |
While mercury electrodes may offer slightly superior detection limits in some configurations [19], bismuth electrodes provide adequate sensitivity for monitoring lead at concentration levels required for blood lead monitoring, typically with regulatory thresholds in the range of 50-100 μg/L [50].
Experimental Protocols for Lead Determination Using Bismuth Electrodes
Electrode Preparation and Modification
The successful application of bismuth electrodes for lead detection requires careful attention to electrode preparation protocols. Two primary approaches have been established for bismuth film formation:
In Situ Bismuth Film Preparation: This method involves adding bismuth ions directly to the sample solution and simultaneously depositing bismuth and target metals during the accumulation step. Typical conditions utilize 150-250 mg/L Bi(III) in acetate buffer (pH 4.5) with a deposition potential of -1.40 V applied for 250 seconds [53]. The in situ method generally provides better adhesion to the substrate and higher sensitivity for lead and cadmium detection compared to ex situ approaches [53].
Ex Situ Bismuth Film Preparation: This approach involves pre-plating the bismuth film onto the substrate electrode before exposure to the sample solution. The ex situ method typically employs a separate plating solution containing 10â»Â³ M bismuth in acetate buffer (pH 4.0) with application of a negative potential for film formation [19]. Ex situ preparation offers more control over film morphology and composition, with studies showing that additives like sodium citrate produce more homogeneous bismuth films with improved analytical performance for lead determination [56].
Table 2: Research Reagent Solutions for Bismuth Electrode Preparation and Analysis
| Reagent/Solution | Function | Typical Composition/Concentration |
|---|---|---|
| Bismuth Stock Solution | Bismuth film formation | 1000 mg/L from Bi(NO₃)₂·4H₂O [53] |
| Acetate Buffer | Supporting electrolyte, pH control | 0.1 M, pH 4.0-4.5 [19] [53] |
| Sodium Sulphate | Background electrolyte | 0.5 M in acetate buffer [19] |
| Dimethylglyoxime (DMG) | Complexing agent for certain metals | Specific concentration varies [52] |
| Sodium Citrate | Film homogenizing agent | Added to plating solution [56] |
Analytical Measurement Protocol
The determination of lead using bismuth electrodes typically follows a well-established stripping voltammetry workflow:
The specific experimental parameters for lead detection include:
Preconcentration/Accumulation Step: Applied potential of -1.10 V to -1.50 V versus Ag/AgCl for 100-250 seconds with solution stirring [53]. This step simultaneously deposits lead and bismuth onto the electrode surface, forming the bismuth-lead alloy.
Equilibrium Period: 15-second quiet time after accumulation to allow for current stabilization [53].
Stripping Step: Application of differential pulse anodic stripping voltammetry (DPASV) or square wave anodic stripping voltammetry (SWASV) with the following typical parameters:
- Potential range: -1.0 V to -0.2 V
- Modulation amplitude: 25 mV
- Step potential: 1.95 mV
- Scan rate: 10 mV/s [53]
Electrode Regeneration: A conditioning step at +0.3 V for 30 seconds under stirring effectively removes residual bismuth and target metals from the electrode surface, preparing it for subsequent measurements [53].
Application to Blood Lead Monitoring: Considerations and Methodologies
Sample Preparation Approaches
The accurate determination of lead in blood samples requires careful sample preparation to eliminate matrix effects and potential interferences:
Acid Digestion: Blood samples typically undergo digestion with concentrated nitric acid to destroy organic matrices and release protein-bound lead [50].
Dilution with Supporting Electrolyte: Direct dilution of digested samples with appropriate supporting electrolyte (e.g., acetate buffer, pH 4.5) provides a simplified approach suitable for bismuth electrode analysis [50].
Standard Addition Methodology: To compensate for matrix effects, the standard addition method is strongly recommended, where known increments of lead standard are added to the sample solution [19].
Overcoming Analytical Challenges in Blood Analysis
Blood matrices present unique challenges for electrochemical analysis, including complex composition and potential interferences:
Decontamination Protocols: Strict protocols must be implemented to prevent environmental contamination during sample preparation and analysis, as lead is ubiquitous in laboratory environments [50].
Interference Management: The presence of surface-active compounds in blood digests may affect electrode performance. The bismuth electrode's relative insensitivity to such interferences represents a significant advantage over other electrode materials [51].
Validation with Certified Reference Materials: Method accuracy should be verified using certified blood reference materials with known lead content to ensure analytical reliability [50].
Comparative Analysis of Electrode Substrates for Bismuth Films
Bismuth films can be deposited on various substrate electrodes, each offering distinct advantages for specific applications:
Table 3: Comparison of Substrate Electrodes for Bismuth Films in Lead Detection
| Substrate Electrode | Advantages | Limitations | Reported LOD for Pb(II) |
|---|---|---|---|
| Pencil Lead Graphite | Inexpensive, easily renewable, wide availability, low background current [53] | Fragile, requires careful assembly | 11.5 μg/L [53] |
| Screen-Printed Carbon | Disposable, miniaturizable, suitable for mass production [19] [54] | Higher cost per measurement, lower conductivity in some cases | Not specified, but successful in tap water analysis [54] |
| Glassy Carbon | Excellent mechanical stability, well-established surface renewal protocols [51] | Higher cost, requires polishing between measurements | Similar to other substrates [51] |
| Carbon Tape | Nanostructured surface, disposable, low-cost [55] | Lower conductivity may influence bismuth modification | 2 μg/L [55] |
| Paper-Based Carbon | Extremely low-cost, easy disposal, hydrophilic properties [19] | Lower sensitivity compared to conventional electrodes | ~0.1 μg/mL (100 μg/L) [19] |
Bismuth-based electrodes represent a viable, environmentally friendly alternative to traditional mercury electrodes for the determination of lead in blood samples. The analytical performance of bismuth film electrodes approaches that of mercury-based systems while eliminating the toxicity concerns associated with mercury handling and disposal. The methodology offers adequate sensitivity for monitoring blood lead levels at clinically relevant concentrations, with detection limits typically in the low μg/L range [53] [50].
Future developments in bismuth electrode technology will likely focus on nanomaterial enhancements to improve sensitivity further, integration with miniaturized portable systems for point-of-care testing, and expansion to multiplexed detection panels for simultaneous determination of multiple heavy metals in clinical samples [51]. The successful application of bismuth electrodes in environmental water analysis [54] provides a strong foundation for their adaptation to biological matrices like blood, supporting their potential as core technology in next-generation clinical monitoring systems for toxic metals.
The accurate quantification of mercury species in biological tissues represents a significant challenge in environmental toxicology and public health research. This challenge is particularly acute in the analysis of breast milk, a complex matrix where the determination of trace levels of total mercury (THg) and methylmercury (MeHg) is crucial for assessing infant exposure risks. The methodological framework for mercury analysis has evolved substantially, with advanced mercury analyzers emerging as key tools alongside other analytical techniques. Within the broader context of research seeking alternatives to mercury electrodes in stripping analysis, the application of direct mercury analyzers demonstrates how modern instrumentation can provide precise measurements while aligning with the principles of green chemistry by eliminating mercury-based analytical processes.
This case study examines the performance of advanced mercury analyzers for determining mercury species in breast milk, comparing their operational parameters, sensitivity, and practical applicability against other established techniques such as Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Gas Chromatography-Mass Spectrometry (GC-MS). By evaluating experimental data from multiple studies, we provide researchers with a comprehensive comparison to inform methodological selection for mercury speciation analysis in complex biological matrices.
Experimental Protocols and Analytical Techniques
Direct Mercury Analysis via Advanced Mercury Analyzer
The Advanced Mercury Analyzer (AMA) represents a sophisticated approach for direct determination of total mercury without extensive sample preparation. This methodology is based on atomic absorption spectrometry with thermal decomposition of samples followed by amalgamation of mercury vapour [57] [58]. In application to breast milk analysis, the typical protocol involves placing an aliquot of approximately 0.2 mL of milk directly into the instrument's sample boat [58]. The sample undergoes thermal decomposition in a combustion tube at approximately 650°C under an oxygen atmosphere, which converts all mercury forms to elemental mercury vapour.
The released mercury is then carried by oxygen to a catalytic tube where impurities are removed, followed by amalgamation on a gold collector. The amalgamator is subsequently heated to release mercury vapour, which is quantified by atomic absorption spectrophotometry at 253.7 nm [57]. This method completely avoids wet chemistry digestion, significantly reducing sample preparation time and potential contamination. The quantification is typically performed using a calibration curve method, with excellent linearity demonstrated in the range of interest for breast milk analysis (Figure 2) [58].
Chromatographic Techniques for Mercury Speciation
For methylmercury speciation, the most validated protocol combines high-performance liquid chromatography with inductively coupled plasma mass spectrometry (HPLC-ICP-MS) with isotope dilution [57]. The sample preparation involves a multi-step extraction: an aliquot of breast milk (typically 2 mL) is spiked with an isotopically enriched (^{198})Hg-enriched methylmercury internal standard, then mixed with HCl and NaBr solutions [58]. The mixture is extracted twice with toluene, and the combined organic extract undergoes back-extraction with L-cysteine aqueous solution. The final analysis is performed by HPLC-ICP-MS, typically using a reversed-phase chromatographic column with a mobile phase containing L-cysteine [58].
Alternative methods for MeHg determination include static headspace GC-MS, which incorporates microwave acid digestion followed by aqueous phase NaBEt(_4) ethylation and detection via single quadrupole mass spectrometry [59] [60]. This approach has demonstrated effectiveness in complex biological matrices, with the advantage of distinguishing between different mercury species through chromatographic separation.
Supplementary Analytical Approaches
Other techniques employed in mercury analysis include Cold Vapor Atomic Absorption Spectrometry (CV-AAS), which has been used in several breast milk studies [61] [62]. This method typically requires sample digestion with strong acids to release mercury, followed by reduction to elemental mercury and detection by AAS. While this technique is widely accessible and cost-effective, it generally offers higher detection limits and less specificity for mercury speciation compared to more advanced instrumentation.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) without chromatographic separation can be used for total mercury determination, offering excellent sensitivity but requiring complete sample digestion and matrix separation to avoid interference [57]. The figures of merit for this technique are comparable to AMA, though operational costs are typically higher.
Figure 1: Comparative workflows for mercury analysis in breast milk using AMA for total mercury and HPLC-ICP-MS for methylmercury speciation
Comparative Performance Data
Analytical Figures of Merit
Table 1: Performance comparison of analytical methods for mercury determination in breast milk
| Analytical Parameter | AMA | ICP-MS | HPLC-ID-ICP-MS (MeHg) | CV-AAS | GC-MS (MeHg) |
|---|---|---|---|---|---|
| Limit of Detection (LOQ) | 0.22 µg/kg wet weight [57] | Comparable to AMA [57] | 0.16 µg/kg wet weight [57] | Higher than AMA [62] | 0.7 µg/kg [59] |
| Repeatability (RSD) | 2-11% [57] | 3-9% [57] | 3-8% [57] | 5-15% [62] | ≤6.8% [59] |
| Intermediate Precision | 4-12% [57] | 5-10% [57] | 4-8% [57] | 8-18% [62] | ≤8% [59] |
| Trueness (Bias) | -0.1 to 9% [57] | -2 to 8% [57] | -4 to 0% [57] | -5 to 12% [62] | 100±2% recovery [59] |
| Sample Throughput | High (minimal preparation) | Medium (digestion required) | Low (extensive preparation) | Medium | Low |
| Speciation Capability | No (THg only) | No (THg only) | Yes (MeHg) | No (THg only) | Yes (MeHg) |
Application to Real Breast Milk Samples
The application of these analytical methods to real breast milk samples reveals important insights about mercury exposure profiles. In a comprehensive study of 180 French breast milk samples, total mercury concentrations determined by AMA ranged from below detection limit to 16.9 µg/kg wet weight, with methylmercury contents ranging from below detection limit to 0.43 µg/kg wet weight [57]. These findings demonstrate the superior sensitivity of modern analytical methods in detecting mercury traces at clinically relevant concentrations.
A German study utilizing CV-AAS reported mean total mercury concentration of 0.90 µg/L in colostrum, declining to <0.25 µg/L after two months of lactation [61]. The correlation between mercury levels and potential exposure sources also varied with analytical methodology, with early postpartum levels showing association with dental amalgam fillings, while later levels correlated more strongly with fish consumption [61].
Research in the Obuasi Municipality of Ghana, employing both AMA for THg and HPLC-ICP-MS for MeHg, demonstrated mean concentrations of 0.4043 µg/L and 0.1829 µg/L for total and methylmercury, respectively [58]. The hazard quotients evaluated in this study indicated potential health concerns for one-month-old infants, highlighting the practical application of these analytical methods in public health risk assessment.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key research reagents and materials for mercury analysis in biological samples
| Reagent/Material | Function | Application Example |
|---|---|---|
| Advanced Mercury Analyzer (AMA) | Direct quantification of total mercury via thermal decomposition, amalgamation, and AAS | Determination of THg in breast milk without sample digestion [57] [58] |
| ICP-MS with HPLC coupling | High-sensitivity detection and speciation of mercury compounds | Separation and quantification of MeHg in breast milk extracts [57] [58] |
| Isotopically Enriched Standards ((^{201})Hg-MeHg, (^{198})Hg-MeHg) | Internal standards for isotope dilution quantification, improving accuracy and precision | Correction for matrix effects and recovery losses in MeHg determination [59] |
| Sodium Tetraethylborate (NaBEt(_4)) | Derivatization agent for conversion of ionic mercury to volatile forms | Ethylation of MeHg for headspace GC-MS analysis [59] |
| L-Cysteine Solution | Complexing agent for selective extraction and stabilization of mercury species | Back-extraction of MeHg from organic solvents in sample preparation [58] |
| Certified Reference Materials | Quality control and method validation | SRM-2974a (mussel tissue), SRM-1946 (fish tissue) for accuracy verification [59] |
| GeA-69 | GeA-69, MF:C20H16N2O, MW:300.4 g/mol | Chemical Reagent |
| GFB-8438 | GFB-8438, CAS:2304549-73-1, MF:C16H14ClF3N4O2, MW:386.7592 | Chemical Reagent |
Method Selection Guidelines for Research Applications
Matrix-Specific Considerations
Breast milk presents particular analytical challenges due to its high fat and protein content, which can interfere with mercury determination and speciation. Based on comparative studies, AMA demonstrates significant advantages for total mercury determination in this matrix by avoiding the incomplete digestions and mercury losses that can occur with conventional acid digestion methods [57]. The minimal sample preparation reduces contamination risks and improves throughput for epidemiological studies requiring analysis of large sample sets.
For speciation analysis, the HPLC-ID-ICP-MS method provides exceptional accuracy and sensitivity for methylmercury determination, though with considerably more complex sample preparation [57]. The isotope dilution approach specifically addresses potential matrix effects and extraction inefficiencies, making it particularly suitable for research requiring high confidence in MeHg quantification at trace levels.
Practical Implementation Factors
Beyond technical performance, practical considerations influence method selection. The AMA system provides rapid analysis with minimal reagent consumption and waste generation, aligning with green chemistry principles [63]. In contrast, chromatographic methods require significant solvent use and generate more chemical waste, though they provide speciated information essential for exposure assessment.
Throughput requirements also guide method selection. AMA can typically analyze dozens of samples per day with minimal preparation, while speciation analysis via HPLC-ICP-MS processes far fewer samples due to extensive preparation and longer analysis times. For large cohort studies, this throughput difference can significantly impact project timelines and costs.
Figure 2: Decision framework for selecting appropriate analytical methods based on research requirements
The comparative analysis of advanced mercury analyzers against alternative methodologies demonstrates their significant value for total mercury determination in breast milk and other complex biological matrices. The AMA platform specifically offers compelling advantages in terms of minimal sample preparation, high throughput, reduced contamination risk, and excellent sensitivity with detection limits sufficient for public health monitoring. For research requiring mercury speciation, the combination of HPLC with ID-ICP-MS provides unparalleled accuracy and sensitivity for methylmercury quantification, despite more extensive sample preparation requirements.
This methodological landscape continues to evolve, with ongoing developments in green analytical chemistry promoting techniques that reduce or eliminate mercury use in analytical processes [63]. The principles demonstrated in this case study – specifically the movement toward direct analysis that minimizes chemical reagents – align with broader trends in analytical chemistry while providing robust tools for assessing environmental exposures and protecting vulnerable populations such as nursing infants.
Maximizing Performance: Overcoming Matrix Effects and Optimizing Mercury-Free Methods
Optimizing Deposition Potential and Time for Maximum Pre-concentration
Electrochemical stripping analysis is a powerful trace analytical technique renowned for its high sensitivity, with detection limits in the order of 10â»Â¹â° to 10â»Â¹Â² mol Lâ»Â¹ [20]. Its fundamental principle involves a two-step process: a pre-concentration step where target analytes are accumulated onto the surface of a working electrode, followed by a stripping step where the accumulated species are measured via a voltammetric or potentiometric scan [14] [20]. The critical importance of the pre-concentration phase cannot be overstated, as its efficiency directly governs the overall sensitivity and detection limits of the method. Within this phase, deposition potential and deposition time emerge as the two most pivotal parameters requiring meticulous optimization. The deposition potential determines the thermodynamic driving force for the reduction and deposition of metal ions, while the deposition time controls the amount of analyte accumulated on the electrode surface [20].
This guide is framed within the broader context of ongoing research into alternatives to traditional mercury electrodes. For decades, mercury electrodes were the cornerstone of stripping analysis due to their exceptional reproducibility, wide cathodic potential window, and ability to form amalgams with many metals [14] [19]. However, concerns over mercury's toxicity and the associated operational hazards have spurred the scientific community to seek safer, more environmentally friendly alternatives [19]. This comparison guide objectively evaluates the performance of the most prominent mercury alternatives—bismuth, carbon, and gold-based electrodes—with a specific focus on the optimization strategies for deposition potential and time to achieve maximum pre-concentration efficiency.
Fundamental Principles of Pre-concentration
In anodic stripping voltammetry (ASV), the most common form of stripping analysis, the pre-concentration step involves the electrochemical reduction of metal ions (Mnâº) to their metallic state (Mâ°) at a suitable deposition potential (E_dep), which is applied to the working electrode:
Mn⺠+ ne⻠→ MⰠ[20]
The deposited metals are subsequently stripped back into solution during the anodic potential scan, generating a current peak for each metal. The height of this peak is proportional to the concentration of the metal in the sample solution [20].
The relationship between deposition parameters and analytical signal is governed by mass transport and electrochemical kinetics. A more negative Edep provides a greater overpotential, driving faster deposition. However, if it is too negative, it may cause co-reduction of interfering species or hydrogen evolution, increasing background noise [19]. Deposition time (tdep) directly influences the amount of analyte accumulated; longer times generally yield higher signals but can lead to electrode saturation, non-linear calibration curves, and longer analysis times [20]. The optimal balance is a deposition potential that is selective and a deposition time that provides sufficient analyte accumulation without surface overloading.
Comparative Analysis of Electrode Materials
The choice of working electrode material fundamentally influences the optimal deposition parameters, sensitivity, and applicability of the stripping analysis method. The following section provides a detailed, data-driven comparison of the primary alternatives to mercury electrodes.
Table 1: Performance Comparison of Electrode Materials for Stripping Analysis
| Electrode Material | Optimal Deposition Potential Range (vs. Ag/AgCl) | Typical Deposition Time Range | Key Advantages | Primary Limitations |
|---|---|---|---|---|
| Mercury Film Electrode (MFE) | -0.9 V to -1.2 V (for Cd, Pb, Cu) [19] | 60 - 600 s [20] | High sensitivity; well-defined stripping signals; wide negative potential window [14] [19] | High toxicity; limited anodic potential window [19] |
| Bismuth Film Electrode (BiFE) | -1.0 V to -1.4 V [19] | 60 - 300 s [19] | Low toxicity; environmentally friendly; wide operational potential window; well-defined stripping peaks [19] | Not suitable for Cu(II) determination; performance can be pH-dependent [19] |
| Bare Carbon Electrode | Variable, highly analyte-dependent | 120 - 600 s [14] | Mercury-free; wide anodic potential window; low cost [14] | Generally lower sensitivity for metal ions; prone to surface fouling [14] |
| Gold Film Electrode | +0.1 V to -0.5 V (for As, Hg) [64] | 30 - 300 s [64] | Excellent for elements forming intermetallic compounds with Au (e.g., As); high sensitivity [64] | Limited cathodic potential window; susceptible to intermetallic compound formation [64] |
Table 2: Quantitative Analytical Performance for Trace Metal Detection
| Electrode Material | Analyte | Limit of Detection (µg/mL) | Linear Range (µg/mL) | Supporting Electrolyte |
|---|---|---|---|---|
| Mercury Film on Paper Carbon [19] | Cd(II) | 0.4 | 0.1 - 10 | Acetate Buffer pH 4.0 |
| Pb(II) | 0.1 | 0.1 - 10 | Acetate Buffer pH 4.0 | |
| In(III) | 0.04 | 0.1 - 10 | Acetate Buffer pH 4.0 | |
| Cu(II) | 0.2 | 0.1 - 10 | Acetate Buffer pH 4.0 | |
| Bismuth Film on Paper Carbon [19] | Cd(II) | Similar to MFE, but not fully quantified | 0.1 - 10 | Acetate Buffer pH 4.0 |
| Pb(II) | Similar to MFE, but not fully quantified | 0.1 - 10 | Acetate Buffer pH 4.0 | |
| In(III) | Similar to MFE, but not fully quantified | 0.1 - 10 | Acetate Buffer pH 4.0 | |
| Cu(II) | Not applicable | Not determinable | Acetate Buffer pH 4.0 |
Key Insights from Comparative Data
- Bismuth as a Viable Mercury Replacement: For simultaneous determination of Cd(II), Pb(II), and In(III), bismuth film electrodes (BiFEs) demonstrate performance comparable to mercury films in terms of linear range, establishing them as a robust, eco-friendly alternative for many applications [19].
- Material-Specific Limitations: The inability of the BiFE to determine Cu(II), as noted in [19], highlights a critical limitation. This is often attributed to the formation of intermetallic compounds between copper and bismuth or competitive deposition, underscoring that electrode choice is inherently analyte-specific.
- Sensitivity Trade-offs: The excellent detection limits achieved by mercury film electrodes (MFEs), as low as 0.04 µg/mL for In(III), reaffirm their historical status as a benchmark for sensitivity [19]. While BiFEs approach this performance, MFEs may still be preferred in scenarios demanding the ultimate detectability.
Experimental Protocols for Parameter Optimization
This section outlines standardized experimental methodologies for determining the optimal deposition potential and time for a given electrode-analyte system.
Generalized Optimization Procedure
A systematic approach for optimizing deposition potential (Edep) and time (tdep) is crucial for method development.
Diagram: Workflow for Optimizing Deposition Parameters
Protocol 1: Optimizing Deposition for a Bismuth Film Electrode
This protocol is adapted from studies using paper-based carbon electrodes modified with bismuth films for heavy metal detection [19].
- Equipment & Reagents: Potentiostat; Screen-printed carbon card or glassy carbon electrode as substrate; Bi(III) stock solution (e.g., 10â»Â³ M in acetate buffer); Standard solutions of target metals (Cd(II), Pb(II), etc.); Acetate buffer (0.1 M, pH 4.0) with 0.5 M Naâ‚‚SOâ‚„ as supporting electrolyte [19].
- Step-by-Step Method:
- Electrode Preparation (Ex Situ Bi Film Plating): Place the working electrode in a plating solution containing 10â»Â³ M Bi(III) in acetate buffer. Apply a deposition potential of -1.0 V to -1.2 V vs. Ag/AgCl for 60-120 seconds under stirring to electrodeposit a uniform bismuth film [19].
- Analyte Pre-concentration: Transfer the BiFEs to a cell containing the sample/standard solution in acetate buffer. While stirring, apply a tested deposition potential (e.g., varying from -0.9 V to -1.4 V) for a fixed time (e.g., 120 s) [19].
- Stripping Measurement: After a quiet time of 10-15 seconds, record an anodic stripping voltammogram using a square-wave or differential pulse waveform. The peak currents for each metal are measured.
- Data Analysis: Plot the stripping peak current (Ip) of each target metal versus the applied deposition potential. The optimal Edep is the value that yields the maximum Ip without excessive background current.
- Time Optimization: Repeat steps 2-4, fixing Edep at the optimized value and varying the deposition time (e.g., 30, 60, 120, 240, 300 s). Plot Ip vs. tdep to establish the relationship and select a time that offers a strong signal without leading to signal saturation.
Protocol 2: On-Line Flow-Based Optimization
For automated systems, optimization can be efficiently performed in a flow-injection or sequential-injection analysis (FIA/SIA) manifold [64].
- Equipment: Flow injection or sequential injection system with multi-port selection valve and peristaltic pump; Electrochemical flow-cell (wall-jet or thin-layer design); Tubing and connectors [64].
- Step-by-Step Method:
- Manifold Setup: Configure the flow manifold to allow sequential selection and pumping of the plating solution, sample/standard, and rinsing solution through the electrochemical cell [64].
- Automated Deposition & Stripping: Program the potentiostat and flow system to execute a continuous cycle: (a) Electrode cleaning, (b) Bi film plating (if applicable), (c) Analyte pre-concentration at a specific Edep and tdep (with solution flowing), (d) Medium exchange (optional), (e) Stripping step, (f) Rinsing [64].
- Parameter Screening: Use the automated system to rapidly screen a wide range of Edep and tdep values across multiple cycles. The strict control of timing and hydrodynamics in flow systems leads to highly reproducible data for optimization [64].
The Scientist's Toolkit: Essential Research Reagent Solutions
The following table details key reagents and materials essential for experiments in optimizing deposition for stripping analysis.
Table 3: Essential Reagents and Materials for Deposition Optimization Studies
| Reagent/Material | Function in Experiment | Example Application & Notes |
|---|---|---|
| Bismuth(III) Salt (e.g., Bi(NO₃)₃) | Source for forming bismuth film electrodes (ex situ or in situ) [19] | Used at ~10â»Â³ M in acetate or acetate buffer for plating. A more environmentally friendly alternative to mercury. |
| Acetate Buffer (pH ~4.0) | Common supporting electrolyte for heavy metal analysis [19] | Provides optimal pH for deposition of many metals (e.g., Cd, Pb, Zn) and stable potential window. Often used with 0.5 M Naâ‚‚SOâ‚„. |
| Mercury(II) Acetate | Source for forming mercury film electrodes [19] | Used at ~10â»Â³ M in HCl for plating. Requires careful handling and disposal due to high toxicity. |
| Standard Metal Solutions | Calibration and method development | Certified standard solutions of target analytes (e.g., 1000 µg/mL Cd, Pb, Cu) for preparing calibration standards [19]. |
| Screen-Printed Electrodes (SPEs) | Disposable, low-cost electrode substrates | Ideal for decentralized analysis. Carbon SPEs can be modified with Bi or Hg films [19]. |
| Nitrogen Gas | Deoxygenation of solutions | Removes dissolved oxygen, which can interfere via reduction peaks, especially at negative potentials. |
| Fitusiran | Fitusiran (Qfitlia) | Fitusiran is an antithrombin-directed siRNA for prophylaxis in hemophilia A or B. Qfitlia is a prescription drug, not for RUO. |
The optimization of deposition potential and time remains a cornerstone of developing highly sensitive and reliable stripping analysis methods. As the field progressively moves away from mercury electrodes, understanding the unique electrochemical behavior of alternative materials like bismuth, carbon, and gold is paramount. This guide has demonstrated that while bismuth film electrodes stand out as a premier substitute for mercury in many applications, offering an excellent balance of performance, low toxicity, and ease of use, the optimal deposition parameters are inherently linked to the specific electrode-analyte system.
Future trends point towards the increased integration of these optimized sensors into automated, on-line monitoring systems [64] and the use of low-cost, disposable substrates such as paper-based electrodes [19]. Furthermore, the exploration of novel nanostructured materials and chemical modifications of electrode surfaces promises to further enhance pre-concentration efficiency, potentially reducing the required deposition times and pushing detection limits to even lower levels. The systematic, data-driven approach to optimization outlined in this guide provides a foundational framework that will remain critical as these new generations of electrode materials continue to evolve.
Selecting the Right Supporting Electrolyte and pH for Complex Biological Matrices
In stripping voltammetry for complex biological matrices, the selection of supporting electrolyte and pH represents a critical methodological parameter that directly governs analytical sensitivity, selectivity, and reproducibility. This decision profoundly influences multiple aspects of the electrochemical system: it dictates the speciation of both target analytes and potential interferents, controls the charge and reactivity of the electrode-solution interface, and impacts the fundamental thermodynamics and kinetics of the electrode processes. The movement toward mercury-free electroanalysis, driven by environmental and safety concerns, has further amplified the importance of optimized electrolyte conditions, as alternative electrode materials often exhibit narrower potential windows and greater susceptibility to surface fouling in biological fluids.
The broader thesis of comparing mercury electrode alternatives necessitates a thorough understanding of how electrolyte composition and pH interact with different electrode materials to affect the determination of biologically relevant analytes, from metal ions to organic pharmaceuticals. This guide provides an objective comparison of performance across different supporting electrolyte systems, supported by experimental data from the research literature, to empower researchers in selecting optimal conditions for their specific analytical challenges in drug development and bioanalysis.
Fundamental Principles: Electrolyte and pH Effects on Electrode Processes
The supporting electrolyte in voltammetric experiments serves three primary functions: (1) to carry current and minimize ohmic (iR) drop, (2) to control the ionic strength and thereby maintain constant activity coefficients, and (3) to fix the pH at the electrode surface within a defined range. In complex biological matrices, these functions become particularly crucial as the electrolyte must overcome the variable conductivity of the sample itself and suppress the migration current of target analytes amidst numerous competing ions.
The pH of the electrolyte system exerts profound effects on electrochemical measurements through multiple mechanisms. For metal ion determination, pH influences hydrolysis and complexation equilibria, which can alter formal potentials and deposition efficiency. For organic analytes, pH directly determines the speciation of acidic and basic functional groups, affecting both their adsorption behavior and their redox potentials. Furthermore, pH controls the hydrogen evolution reaction, which defines the cathodic potential window limit, particularly critical for less noble electrode materials like bismuth or antimony.
The electrical double-layer structure, which varies with both supporting electrolyte concentration and pH, significantly influences the kinetics of electrode reactions. The Frumkin correction for adsorption isotherms accounts for these effects, which become particularly significant in high-ionic-strength biological matrices where double-layer compression occurs.
Comparative Performance of Supporting Electrolyte Systems
Different supporting electrolyte systems offer distinct advantages and limitations for analysis in biological matrices. The table below summarizes key electrolytes with their optimal pH ranges and applications:
Table 1: Comparison of Supporting Electrolyte Systems for Biological Matrices
| Electrolyte | Optimal pH Range | Key Applications | Advantages | Limitations |
|---|---|---|---|---|
| Acetate Buffer | 3.5-5.8 | Metal determination (Ga, Pb, Cd), organic compound adsorption studies | Weakly adsorbed ions minimize competitive adsorption; suitable for physiological pH simulation [65] | Limited buffering capacity at extremes of pH range |
| Britton-Robinson Buffer | 2.0-12.0 | Pharmaceutical compounds (e.g., closantel), broad screening studies | Wide buffering range; versatile for multiple analyte classes | Complex composition may interact with some analytes |
| Ammonium Acetate-HCl Buffer | ~4.5 | Adsorptive stripping voltammetry of metal complexes | Controlled complexation for selective determination | Limited temperature stability |
| Phosphate Buffer | 6.0-8.0 | Biological samples, enzyme-based sensors | Physiological relevance; biocompatibility | May form insoluble complexes with some metal ions |
The selection of an appropriate electrolyte-ph combination requires consideration of both the target analyte's properties and the matrix composition. For instance, the determination of gallium(III) using adsorptive stripping voltammetry with catechol as a complexing agent achieves optimal sensitivity in 0.1 M acetate buffer at pH 4.8 [7], where the Ga(III)-catechol complex exhibits optimal adsorption and electrochemical behavior. Similarly, the electrochemical determination of the veterinary drug closantel shows well-defined reduction peaks in Britton-Robinson buffer at pH 7.0 [66], highlighting how neutral pH conditions may be preferable for certain pharmaceutical compounds.
Electrode Material Performance Across Different Electrolyte Conditions
The interaction between electrode material and supporting electrolyte fundamentally determines analytical performance in stripping analysis. Different electrode materials exhibit distinct potential windows, adsorption characteristics, and interfacial properties that must be matched with appropriate electrolyte conditions.
Table 2: Electrode-Electrolyte Synergies in Stripping Analysis
| Electrode Material | Optimal Electrolyte/pH | Detection Limits | Biological Matrix Applications | Key Performance Characteristics |
|---|---|---|---|---|
| Hanging Mercury Drop Electrode (HMDE) | 0.02 M NaClOâ‚„, 0.005 M CH₃COOH, pH 3.2 [7] | 5.7×10â»Â¹Â¹ M for metals [7] | Multi-metal analysis in digested soils [13] | Excellent reproducibility, wide cathodic potential window, renewable surface [13] |
| Mercury Film Electrodes | 0.1 M acetate buffer, pH 4.8 [7] | 3.6×10â»Â¹â° M for Ga(III) with catechol [7] | Trace metal detection with faster stirring capabilities [67] | Larger surface area/volume ratio, faster deposition, sharper peaks [67] |
| Bismuth Film Electrodes | 0.1 M HCl or acetate buffer, pH ~4.5 | Sub-nanomolar for heavy metals [22] | Disposable sensors for environmental and biological monitoring | "Green" alternative, low toxicity, performance approaching mercury [22] |
| Silver Amalgam Film Electrodes | Britton-Robinson buffer, pH 7.0 [66] | 1.1×10â»â¸ M for closantel [66] | Pharmaceutical analysis in veterinary formulations | Mechanically renewable surface, wide potential window, reduced mercury content [66] |
The data reveal that while mercury-based electrodes continue to offer exceptional sensitivity and well-characterized electrolyte interactions, the emerging "green" alternatives demonstrate competitive performance when paired with appropriately optimized electrolyte systems. For instance, bismuth film electrodes in 0.1 M HCl or acetate buffer achieve detection limits approaching those of mercury electrodes for heavy metals like cadmium and lead [22], making them viable alternatives for many biological applications where toxicity concerns preclude mercury use.
Experimental Protocols for Method Optimization
Standard Method for Supporting Electrolyte Screening
- Prepare stock solutions of the target analyte at a concentration 10-fold higher than the expected detection limit.
- Prepare candidate electrolyte systems covering a range of pH values and buffer compositions. Common systems include acetate (pH 3.6-5.6), phosphate (pH 6.0-8.0), Britton-Robinson (pH 2.0-12.0), and ammonia-ammonium chloride (pH 9.0-10.0).
- Mix equal volumes of analyte stock solution with each electrolyte system in the electrochemical cell.
- Record voltammograms using identical instrumental parameters (deposition time, potential, scan rate) across all electrolyte systems.
- Evaluate responses based on peak current, peak shape, background current, and potential separation in multicomponent systems.
- Select the optimal system that provides the highest signal-to-noise ratio with minimal interference.
pH Optimization Protocol for Adsorptive Stripping Voltammetry
- Prepare the selected supporting electrolyte at a constant concentration (typically 0.1 M).
- Adjust pH in increments of 0.5 pH units across the usable range of the buffer system.
- Add complexing agent if required for adsorptive accumulation (e.g., catechol for Ga(III) determination).
- Perform measurements with constant accumulation time and potential.
- Plot peak current versus pH to identify the optimum. A bell-shaped curve typically results, reflecting the balance between complex stability, adsorption efficiency, and electrochemical reactivity.
Interference Testing in Complex Matrices
- Spike the optimized system with potential interferents commonly found in biological matrices (e.g., proteins, amino acids, metal ions, reducing agents).
- Evaluate signal suppression/enhancement by comparing responses with and without interferents.
- Implement matrix modification strategies if interference exceeds acceptable limits (e.g., UV digestion, standard addition, separation techniques).
Diagram: Electrolyte and pH Optimization Workflow
The following diagram illustrates the systematic approach to optimizing supporting electrolyte and pH conditions for electrochemical analysis in complex biological matrices:
The Scientist's Toolkit: Essential Research Reagent Solutions
Successful implementation of stripping voltammetry in biological matrices requires careful selection of research reagents and materials. The following table outlines essential solutions and their functions:
Table 3: Essential Research Reagent Solutions for Electroanalysis
| Reagent Solution | Composition | Primary Function | Application Notes |
|---|---|---|---|
| Supporting Electrolyte Stock | 1.0 M buffer solution (acetate, phosphate, etc.) | Provide ionic strength, control pH, minimize iR drop | Filter (0.45 μm) and degas before use; prepare fresh weekly |
| Standard Metal Solutions | 1000 ppm metal ions in 1% HNO₃ | Calibration standards for metal determination | Use trace metal grade acids; dilute daily from stock |
| Complexing Agent Solution | 0.01-0.1 M ligand (catechol, cupferron, etc.) | Form electroactive complexes for AdSV | Prepare fresh daily; protect from light if photolabile |
| Matrix Modifier | UV digestion reagents, masking agents | Reduce interference in complex biological samples | Concentration must be optimized for each matrix type |
| Electrode Cleaning Solution | 0.1 M HNO₃, ethanol, alumina polish | Maintain electrode surface reproducibility | Follow manufacturer recommendations for specific electrodes |
Comparative Experimental Data: Mercury vs. Alternative Electrodes
Direct comparison of experimental data reveals the relative performance of different electrode-electrolyte systems. Recent studies demonstrate that while mercury electrodes continue to provide exceptional sensitivity, alternative materials show promising performance in optimized conditions:
Table 4: Performance Comparison Across Electrode-Electrolyte Systems
| Analyte | Electrode | Supporting Electrolyte | Detection Limit | Linear Range | Reference |
|---|---|---|---|---|---|
| Ga(III) | HMDE | 0.02 M NaClOâ‚„, 0.005 M CH₃COOH, pH 3.2 | 5.7×10â»Â¹Â¹ M | Not specified | [7] |
| Ga(III) | Hg(Ag)FE | 0.1 M acetate buffer, pH 4.8 with catechol | 3.6×10â»Â¹â° M | 1.25×10â»â¹ - 9.0×10â»â¸ M | [7] |
| Ga(III) | PbFE/MWCNT/SGCE | 0.1 M acetate buffer, pH 5.6 with cupferron | 9.5×10â»Â¹â° M | 3.0×10â»â¹ - 4.0×10â»â· M | [7] |
| Closantel | Hg(Ag)FE | Britton-Robinson buffer, pH 7.0 | 1.1×10â»â¸ M | 5.0×10â»â¸ - 1.2×10â»â¶ M | [66] |
| Zn²âº, Cd²âº, Pb²âº, Cu²⺠| HMDE | Acid-digested soil samples | (Sub)nanomolar | Wide linear range | [13] |
| Toxic Elements | BiFE, SbFE, SnFE | Various optimized electrolytes | Nanomolar range | Varies by element | [22] |
The data indicate that properly optimized alternative electrodes can achieve detection limits within one order of magnitude of traditional mercury-based systems, while offering significantly improved environmental and safety profiles. The mercury-silver amalgam film electrode (Hg(Ag)FE) appears particularly promising, combining reduced mercury content with excellent analytical performance [66].
The optimal selection of supporting electrolyte and pH for complex biological matrices requires a systematic approach that balances multiple competing factors. Based on the comparative data presented, the following strategic guidelines emerge:
For broad-spectrum screening of unknown biological samples, begin with Britton-Robinson buffer due to its wide buffering range, which enables initial assessment of pH effects without changing buffer composition.
For trace metal determination, acetate buffers in the pH 4-5 range generally provide optimal performance for both mercury and alternative electrodes, offering sufficient buffering capacity with minimal competitive adsorption.
When using "green" alternative electrodes like bismuth or antimony films, pay particular attention to the cathodic potential window limits, as these materials are more susceptible to hydrogen evolution, especially in strongly acidic media.
For pharmaceutical compounds with specific acid-base properties, match the electrolyte pH to the analyte's pKa to optimize adsorption and electron transfer kinetics.
The continuing evolution of electrode materials and the growing emphasis on environmentally friendly analytical methodologies ensure that optimization of supporting electrolyte and pH conditions will remain a dynamic research area. As new electrode materials emerge, systematic re-evaluation of traditional electrolyte systems will be essential to fully exploit their analytical potential in the challenging environment of complex biological matrices.
Strategies to Minimize Interferences in Protein-Rich Samples like Serum and Milk
Electrochemical stripping analysis is renowned for its high sensitivity in trace metal and biomolecule detection. However, the analysis of protein-rich samples such as serum and milk presents significant challenges due to matrix effects. These effects arise when proteins and other sample components interfere with the analyte signal, compromising accuracy, reproducibility, and sensitivity [68] [69]. Within the broader thesis of finding mercury electrode alternatives for stripping analysis research, this guide objectively compares the performance of different electrodes and methodologies specifically for mitigating interferences in complex biological matrices.
Protein-rich samples can cause fouling of electrode surfaces, compete for adsorption sites, and alter the viscosity and conductivity of the solution [70]. These factors lead to ionization suppression or enhancement in detection systems and reduced analytical performance [69]. This guide compares established and emerging strategies to overcome these hurdles, providing experimental data and protocols to inform method selection for research and development applications.
Comparative Electrode Performance in Complex Matrices
The choice of working electrode is fundamental to the performance of any stripping voltammetric method. While mercury electrodes have historically been the gold standard for many applications, their toxicity has driven the development of alternative materials, especially for biological analysis [13].
Mercury-Based Electrodes
The hanging mercury drop electrode (HMDE) offers excellent reproducibility, a wide cathodic potential window, and the ability to form amalgams with many metal ions [13]. Its renewable surface is particularly advantageous for protein-rich samples, as each new measurement is performed on a fresh, clean surface, minimizing carryover and fouling [13]. In adsorptive potentiometric stripping analysis (PSA), the HMDE effectively addresses hydrogen discharge background problems, enabling convenient quantitation of nucleic acids based on their cytosine and adenine residues, which is a significant advantage for studies of DNA structure and interactions in biological samples [71].
Table 1: Performance Comparison of Electrodes for Analysis in Complex Matrices
| Electrode Type | Key Advantages | Limitations in Protein-Rich Samples | Representative Detection Limits |
|---|---|---|---|
| Hanging Mercury Drop Electrode (HMDE) | - Renewable surface prevents fouling [13]- Wide cathodic potential window [13]- Excellent for amalgam-forming metals [72] | - High toxicity of mercury [13]- Potential surface adsorption of proteins | - tRNA: 30 μg Lâ»Â¹ [71]- Cd, Pb, Cu, Zn in digested soils (via ASV) [13] |
| Mercury Thin-Film Electrode (MTFE) | - Larger surface-to-volume ratio than HMDE [71]- Suitable for flow-mode analysis [71] | - More susceptible to surface fouling than HMDE- Toxicity of mercury | |
| Bismuth-Film Electrode (BiFE) | - Low toxicity [13]- Well-defined stripping peaks for several metals [13] | - Performance can be degraded in highly complex matrices- Limited negative potential window compared to Hg | - Pb, Cd in drinking water (meets WHO guidelines) [72] |
| Gold Electrode | - Useful for Hg, Se, and As analysis [72] | - Irreversible adsorption of some analytes (e.g., mercury) alters surface [73] | |
| Palladium-Based Sensor | - Simple microfabrication [74] | - Relatively new technology, less established | - Mn: 334 nM (18.3 ppb) in borate buffer [74] |
Mercury-Free Solid Electrodes
Driven by the need for less toxic and more robust alternatives, several solid electrodes have been developed.
- Bismuth-Film Electrodes (BiFEs): Bismuth is an environmentally friendly element that forms "fused" multi-metal alloys with heavy metals like Pb, Cd, and Zn. BiFEs have been successfully demonstrated for the analysis of heavy metals in drinking water, achieving detection limits that meet World Health Organization regulations [72]. Their performance in undiluted, protein-rich matrices like serum may require careful sample preparation or matrix-matching calibration.
- Gold Electrodes: Gold is valuable for determining elements that do not form amalgams, such as arsenic and mercury. However, studies show that mercury is not completely removed electrochemically from gold electrodes after analysis, fundamentally altering the electrode's nature through irreversible adsorption [73]. This can be a significant drawback in high-throughput analysis of biological samples.
- Palladium-Based Sensors: Recent research has explored palladium-based microfabricated sensors for point-of-care applications. These sensors integrate with printed circuit board processing and have been used with square wave cathodic stripping voltammetry to determine manganese in natural waters with a limit of detection of 334 nM [74]. Their disposability is a key advantage for avoiding cross-contamination in complex samples.
Methodological Strategies for Interference Minimization
Beyond electrode selection, the analytical strategy itself is critical for reliable results in protein-rich environments.
Sample Preparation and Clean-Up
The primary goal of sample preparation is to remove interfering proteins and other matrix components while maintaining the integrity of the analyte.
- Acid Digestion and UV Irradiation: For the determination of trace metals in biological samples, a simple pretreatment like acidification or UV irradiation is often sufficient prior to voltammetric determination [75]. In a study analyzing soil samples (a complex matrix analogous to biological solids in complexity), a digestion with 5 M HNO₃ was used to extract metals before ASV analysis with an HMDE [13].
- Protein Precipitation and Filtration: For liquid samples like serum and milk, protein precipitation with strong acids or organic solvents followed by filtration is a common approach. In LC-MS, a recognized technique to minimize matrix effects is to use a divert valve to switch the flow from the column to waste during the elution of proteins, thereby preventing them from reaching the ion source [68]. This principle can be adapted in flow-based stripping analysis systems.
- Selective Extraction Techniques: The development of molecularly imprinted polymers (MIPs) promises high selectivity in extraction, offering high recovery percentages and low matrix effects, though this technology is not yet widely commercially available [68].
Calibration Techniques to Compensate for Matrix Effects
Even with extensive clean-up, residual matrix effects often persist. Calibration strategies are therefore essential for accurate quantification.
- Standard Addition Method: This method is highly effective for compensating for matrix effects and is particularly useful when a blank matrix is unavailable, as is the case with native serum or milk [69]. It involves spiking the sample itself with known quantities of the analyte. While standard addition is routine in atomic spectroscopy, its application in LC-MS to compensate for matrix effects is less documented, and it is similarly underutilized in stripping voltammetry for biological samples, representing a significant opportunity for improved accuracy [69].
- Matrix-Matched Calibration: This approach involves preparing calibration standards in a solution that mimics the sample matrix. However, obtaining an appropriate blank matrix for biological fluids like serum is often impossible, and it is challenging to exactly match the matrix of every unknown sample [69].
- Internal Standardization: The use of a stable isotope-labeled internal standard (SIL-IS) is considered the best practice in LC-MS because it co-elutes with the analyte and experiences nearly identical matrix effects [68] [69]. A more accessible, though less perfect, alternative is the use of a co-eluting structural analogue of the analyte [69].
Table 2: Comparison of Calibration Strategies for Matrix Effect Compensation
| Calibration Method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Standard Addition | Known analyte quantities are added directly to the sample [69]. | - Does not require a blank matrix.- Corrects for multiplicative interferences. | - Time-consuming for large batch analysis.- Increases sample preparation workload. |
| Matrix-Matched Calibration | Calibrators are prepared in a matrix similar to the sample [69]. | - Conceptually simple.- Can be accurate if the matrix is well-matched. | - Blank matrix is often unavailable (e.g., for serum).- Impossible to match all sample matrices exactly. |
| Stable Isotope-Labeled Internal Standard (SIL-IS) | A chemically identical, isotopically labeled version of the analyte is added [68] [69]. | - Gold standard; corrects for both preparation and ionization variability.- High accuracy. | - Expensive.- Not always commercially available. |
| Structural Analogue Internal Standard | A compound structurally similar to the analyte is used [69]. | - More readily available and cheaper than SIL-IS. | - May not perfectly mimic analyte behavior in sample preparation or ionization. |
Experimental Protocols for Method Validation
To ensure reliability, any new analytical method must be validated. The following protocols are essential for assessing method performance with protein-rich samples.
Post-Extraction Spike Method for Quantitative ME Assessment
This method, proposed by Matuszewski et al., provides a quantitative measure of matrix effects [68] [69].
- Prepare three sets of samples:
- Set A (Neat Solution): Analyze the analyte dissolved in a pure, simple solvent (e.g., mobile phase).
- Set B (Spiked Post-Extraction): Take a blank matrix (e.g., protein-free serum supernatant), perform the entire sample preparation protocol, and then spike the analyte into the prepared extract.
- Set C (Spiked Pre-Extraction): Spike the analyte into the native blank matrix and then perform the entire sample preparation protocol.
- Analyze all sets using the developed stripping voltammetry method.
- Calculate the matrix effect (ME) and recovery (RE):
Matrix Effect (ME, %) = (Peak Response of Set B / Peak Response of Set A) × 100Recovery (RE, %) = (Peak Response of Set C / Peak Response of Set B) × 100Process Efficiency (PE, %) = (Peak Response of Set C / Peak Response of Set A) × 100 = (ME × RE)/100
A matrix effect value of 100% indicates no suppression or enhancement. Values below 100% indicate suppression, and values above indicate enhancement.
Protocol for ASV with HMDE in Digested Samples
This protocol, adapted from the analysis of digested soils, demonstrates a robust approach for metal analysis in complex, solid-like matrices [13].
- Sample Digestion: Treat the sample (e.g., 20 g of soil or 1 mL of serum) with 100 mL of 5 M HNO₃ for 60 minutes under stirring. For organic-rich samples like milk, additional steps or stronger oxidizing agents may be required.
- Filtration and Dilution: Suction-filter the digested sample. Dilute an aliquot (e.g., 10 µL) with HNO₃ to a final volume of 20 mL in the voltammetric cell.
- Deaeration: Purge the solution with an inert gas like nitrogen for 10 minutes to remove dissolved oxygen.
- Deposition: Apply a deposition potential (e.g., -1.1 V for Zn, Cd, Pb, Cu) for a set time (e.g., 120 s) under stirring to pre-concentrate the metals into the mercury drop.
- Equilibration: Turn off the stirrer and allow the solution to equilibrate for 30 s.
- Stripping: Scan the potential (e.g., from -1.1 V to +0.15 V) using a sensitive technique like Square Wave ASV (SWASV) to strip the metals back into solution and record the current response.
- Data Analysis: Perform baseline subtraction and peak integration. Quantify using the standard addition method for highest accuracy.
The Scientist's Toolkit: Essential Reagent Solutions
The following table details key reagents and materials used in stripping analysis of complex samples, based on the experimental data from the cited literature.
Table 3: Key Research Reagent Solutions for Stripping Analysis
| Reagent/Material | Function/Purpose | Example from Literature |
|---|---|---|
| Hanging Mercury Drop Electrode (HMDE) | Renewable working electrode for trace metal and biomolecule analysis; minimizes fouling [13]. | Simultaneous determination of Zn²âº, Cd²âº, Pb²âº, and Cu²⺠in digested soil samples [13]. |
| Nitric Acid (HNO₃) | Digesting agent for organic matrices and preparation of supporting electrolyte [13]. | Sample digestion protocol using 5 M HNO₃ for 60 minutes [13]. |
| Dimethylglyoxime (DMG) | Analytical ligand for adsorptive stripping determination of elements like Nickel and Cobalt [75]. | Determination of dissolved Ni in coastal waters; added to sample with a pH buffer (HEPES, pH 7.8) [75]. |
| Bismuth Film | Environmentally friendly plating material for mercury-free electrodes [72]. | Detection of lead and cadmium in drinking water using square wave voltammetry [72]. |
| Stable Isotope-Labeled Internal Standard | Ideal internal standard for compensating for matrix effects and losses during sample preparation [68] [69]. | Use of creatinine-d₃ for the LC-MS analysis of creatinine in urine to correct for matrix effects [69]. |
| Catechol | Analytical ligand for simultaneous determination of multiple trace metals (e.g., Cu, Fe, V, U) in a single AdSV measurement [75]. | Application in shipboard analysis of coastal waters for trace metal determination [75]. |
Workflow Diagram for Method Development
The following diagram outlines a logical decision pathway for developing a stripping analysis method for protein-rich samples, incorporating strategies to minimize interferences.
Electrode Activation and Maintenance for Long-Term Reproducibility
In electrochemical research, particularly in stripping analysis for trace metal detection and pharmaceutical development, the reproducibility of electrode performance is not merely a convenience but a fundamental requirement for generating valid, comparable scientific data. Long-term reproducibility ensures that experimental results remain consistent across different testing sessions, laboratories, and operators, thereby validating research findings and enabling reliable industrial process scaling. Electrode activation and systematic maintenance protocols serve as the cornerstone for achieving this reproducibility, especially as the field progressively transitions toward mercury-free electrode alternatives due to environmental and safety concerns.
The challenge of reproducibility is particularly acute in stripping analysis, where minute variations in electrode surface characteristics can significantly impact pre-concentration efficiency and stripping signals. This guide objectively compares the performance characteristics of modern electrode materials, provides detailed activation and maintenance protocols, and presents experimental data to empower researchers in selecting and maintaining electrodes for consistent, long-term analytical performance.
Comparative Analysis of Electrode Materials
Performance Characteristics of Electrode Materials
Table 1: Comparative performance of electrode materials for stripping analysis.
| Electrode Material | Key Advantages | Limitations & Stability Concerns | Reported Longevity / Stability Data | Best Suited Applications |
|---|---|---|---|---|
| Bismuth-Film Coated Electrodes | High hydrogen overvoltage, low toxicity, well-defined stripping signals [76]. | Film stability over repeated cycles, uniformity of plating. | – | Simultaneous determination of Zn(II), Cd(II), Pb(II) [76]. |
| Platinum Black Modified Electrodes | Substantially increased charge injection capacity (CIC) and electroactive area; reduced impedance [77]. | Potential for physical degradation; requires electrochemical validation. | Stable CIC after 7-day continuous stimulation; Coating maintained post-stability testing [77]. | Neural stimulation; applications requiring high CIC [77]. |
| Carbon-Based Electrodes (e.g., Carbon Paste, Glassy Carbon) | Wide potential window; modifiable surface; mercury-free [76] [44]. | Surface fouling; requires specific activation (e.g., Glassy Carbon for Zn analysis) [44]. | – | Trace metal analysis; anodic stripping voltammetry (ASV); adsorptive stripping voltammetry (AdSV) [7]. |
| Dioctyl Phthalate-Based Carbon Paste | Extremely wide cathodic potential window minimizes hydrogen evolution interference [76]. | – | – | Stripping analysis of elements with highly negative reduction potentials (e.g., Zn) [76]. |
Quantitative Electrochemical Performance Data
Table 2: Experimental electrochemical performance data for electrode materials.
| Electrode Material | Charge Storage Capacity (CSC) / Charge Injection Capacity (CIC) | Impedance (at 10 Hz) | Electroactive Area Increase | Key Experimental Conditions |
|---|---|---|---|---|
| Platinum (Uncoated) | CIC: ~21.9 µC cmâ»Â² [77] | – | – | 0.9% saline; biphasic pulse, 250 µs [77]. |
| Platinum Black Coated | CIC: ~64.9 µC cmâ»Â² [77] | Reduced vs. uncoated Pt [77] | Substantial [77] | 0.9% saline; biphasic pulse, 250 µs [77]. |
| Bismuth-Coated Carbon Paste | – | – | – | Simultaneous detection of Cd(II), Pb(II), Zn(II) [76]. |
Experimental Protocols for Electrode Activation and Testing
Standardized Catalyst Screening Protocol (Three-Electrode Cell)
This robust protocol is designed for screening the activity of electrocatalysts like those used in the Oxygen Evolution Reaction (OER) and Hydrogen Evolution Reaction (HER) under conditions closer to a real electrolyzer than a Rotating Disk Electrode (RDE) [78].
Equipment and Reagents [78]:
- Potentiostat: Standard instrument with 5 V and 5 A capability.
- Electrolyte: Aqueous solution of KOH (0.1–1 M concentration), sparged with inert gas (e.g., Argon).
- Electrodes:
- Working Electrode (WE): Conductive substrate (e.g., stainless-steel mesh, carbon paper) coated with catalyst.
- Counter Electrode (CE): Platinum wire/sheet (for PGM catalysts) or graphite rod (for PGM-free catalysts).
- Reference Electrode (RE): Hg/HgO for alkaline solutions.
Step-by-Step Procedure [78]:
- Cell Setup: Fill the electrochemical cell with sparged electrolyte. Assemble the three-electrode setup, ensuring no physical contact between electrodes or their clips.
- Catalyst Conditioning: Condition the catalyst on the WE via initial cycling.
- Linear Sweep Voltammetry (LSV):
- For OER catalysts: Perform LSV between 1.4 and 2.2 V vs. RHE at a scan rate of 10 mV/s.
- For HER catalysts: Perform LSV between 0 and -0.5 V vs. RHE at a scan rate of 10 mV/s.
- Impedance Measurement: Measure the solution resistance between WE and RE via electrochemical impedance spectroscopy (100 kHz to 0.1 Hz). Use this value for IR correction of LSV data.
- Stability Assessment (Bulk Electrolysis): Perform chronoamperometry (CA). Hold at 0 A for 3 s, then at a fixed current density (e.g., 20 mA) for 1 hour, followed by 0 A for 1 s.
Activation of Glassy Carbon Electrodes for Zinc Analysis
For mercury-free analysis of zinc, which has a highly negative reduction potential, Glassy Carbon Electrodes (GCE) require a specific activation process [44]:
- Pre-concentration: In a mercury-free electrolyte of 0.1 M HCl containing 2 ppm Zn²âº, pre-concentrate zinc on the GCE surface at a constant potential of -1400 mV (vs. SCE).
- Stripping: The accumulated zinc is subsequently stripped at approximately -1050 mV (vs. SCE).
- Outcome: This activation process enables a linear relationship between stripping peak area and zinc concentration in the range of 0–2000 ppb [44].
Stability Testing via Continuous Stimulation Paradigm
To evaluate the long-term stability and durability of electrode materials, particularly for high-demand applications like neural stimulation, a continuous testing protocol is employed [77]:
- Pre-characterization: Characterize the electrodes using Cyclic Voltammetry (CV), Electrochemical Impedance Spectroscopy (EIS), and Chronopotentiometric Voltage Transients (VT) to establish baseline performance.
- Continuous Stimulation: Subject the electrodes to a continuous stimulation paradigm for 7 days in a relevant electrolyte (e.g., non-degassed 0.9% saline).
- Apply a biphasic pulse (e.g., cathodic-first, 300 µs phase width) at a high pulse frequency (e.g., 500 Hz).
- The pulse amplitude can be set to a significant percentage (e.g., 90%) of the electrode's predetermined Charge Injection Capacity (CIC).
- Post-Test Analysis: Re-characterize the electrodes using the same techniques (CV, EIS, VT) to quantify any changes in CSC, CIC, impedance, or surface morphology.
The Researcher's Toolkit: Essential Reagents and Materials
Table 3: Key research reagents and materials for electrode activation and maintenance.
| Item Name | Function / Purpose | Example Application / Note |
|---|---|---|
| Potentiostat/Galvanostat | Applies controlled potential/current and measures electrochemical response. | Fundamental instrument for all activation and testing protocols [78] [77]. |
| Hg/HgO Reference Electrode | Provides stable, known reference potential in alkaline media. | Recommended for OER/HER testing in KOH electrolytes [78]. |
| Platinum Wire/Counter Electrode | Serves as the counter electrode to complete the circuit. | Used when the working electrode contains Platinum Group Metals (PGMs) [78]. |
| Graphite Rod Counter Electrode | Serves as an inert counter electrode. | Used for PGM-free working electrodes to avoid contamination [78]. |
| Potassium Hydroxide (KOH) | Common electrolyte for alkaline electrocatalysis (HER/OER). | Use high purity (≥95%); solutions typically 0.1–1 M [78]. |
| Platinum Black Coating | Significantly increases surface area and charge injection capacity of Pt electrodes. | Applied via electrodeposition or sputter coating [77]. |
| Bismuth Coating Solution | Forms a bismuth-film on substrate electrodes for environmentally friendly stripping analysis. | Alternative to mercury-based electrodes [76]. |
Workflow for Electrode Preparation and Maintenance
The following diagram illustrates a generalized, robust workflow for ensuring electrode reproducibility, integrating steps from the cited protocols.
Electrode Reproducibility Workflow
The pursuit of long-term electrode reproducibility hinges on a trifecta of factors: the selection of appropriate, robust materials like platinum black or bismuth-film electrodes; the strict adherence to detailed activation protocols such as those for glassy carbon; and the implementation of rigorous, continuous stability testing. As the field of electroanalysis moves inexorably toward mercury-free alternatives and finds new applications in pharmaceutical API synthesis [79] and neural interfaces [77], the standardization of these activation and maintenance procedures will become increasingly critical. Future developments will likely focus on creating even more durable electrode coatings with self-conditioning properties and establishing universally accepted benchmarking protocols, ultimately ensuring that data reproducibility remains the foundation of electrochemical science.
Electrochemical stripping analysis is a powerful technique for the trace-level quantification of metal ions, prized for its remarkable sensitivity derived from a preconcentration step coupled with electrochemical measurement [15] [80]. For decades, mercury electrodes were the cornerstone of this method due to their excellent electrochemical properties, including a wide cathodic potential range and reproducible renewal surface [22]. However, mercury's significant toxicity and associated legal restrictions have driven the scientific community to develop environmentally friendly alternative electrode materials [22] [80].
This guide objectively compares the analytical performance of the leading "green" electrode materials—bismuth, antimony, tin, and gold—against traditional mercury, with a specific focus on their application in conjunction with the standard addition method for analyzing complex matrices. Accurate quantification in such samples (e.g., environmental waters, biological fluids) is challenging due to the matrix effect, where unknown sample components can enhance or suppress the analytical signal. The standard addition method, which involves adding known quantities of the analyte to the sample, is crucial for compensating for these effects and achieving accurate results [81].
Experimental Protocols for Electrode Preparation and Measurement
Preparation of Mercury-Alternative Electrodes
Bismuth-Film Electrode (BiFE) on Copper Substrate
- Substrate Preparation: Begin with a copper substrate. Polish the surface mechanically if necessary to ensure uniformity.
- Electrodeposition: Immerse the electrode in a plating solution containing bismuth ions (e.g., 20 mM Bi(III) in 0.5 M KBr and 1 M HCl). Apply a constant potential of -0.25 V vs. Ag/AgCl for 300 seconds without stirring to deposit a metallic bismuth film [81]. The film should be visually uniform and adherent.
- Validation: The electrode is ready for use after deposition. Its performance can be validated using a standard solution of Cd²⺠and Pb²⺠[80].
Bismuth-Film Electrode on Screen-Printed Carbon Substrate
- In-Situ Plating: Add bismuth ions directly to the sample solution (typically at a concentration of 200-400 µg/L). Simultaneously deposit the target metals and the bismuth film onto the carbon working electrode by applying a deposition potential (e.g., -1.4 V vs. Ag/AgCl for 60-120 seconds) with solution stirring [22].
- Ex-Situ Plating: Alternatively, plate the bismuth film from a separate plating solution before exposing the electrode to the sample solution. This method offers better control over the film morphology [22].
Antimony and Tin Film Electrodes
- The preparation protocols for antimony film electrodes (SbFEs) and tin film electrodes (SnFEs) are analogous to those for BiFEs, involving the electroreduction of Sb(III) or Sn(II) salts onto a suitable substrate, either ex-situ or in-situ [22].
Gold-Modified Electrodes
- Electroplating: For a gold nanoparticle-modified carbon electrode, electroplate from a solution containing tetrachloroaurate(III) ions ([AuClâ‚„]â»). Apply a constant potential or use potential cycling to deposit gold nanoparticles onto the substrate [22].
- Direct Use: Commercially available screen-printed electrodes with gold or gold nanoparticle-based inks can be used directly without a plating step [22].
General Anodic Stripping Voltammetry Protocol with Standard Addition
The following workflow, applicable to most mercury-free electrodes, details the standard addition method for quantifying metal ions in a complex matrix.
Step-by-Step Procedure:
- Electrode Preparation: Prepare the working electrode (e.g., BiFE, SbFE, Au-SPE) according to the protocols above. Set up the standard three-electrode cell, including the reference (e.g., Ag/AgCl) and counter (e.g., Pt wire) electrodes [15] [82].
- Sample Aliquoting: Divide the unknown sample solution (e.g., river water, plant extract) into several equal-volume aliquots. Typically, four aliquots are used: one unspiked and three spiked with different standard concentrations [80] [81].
- Standard Additions: To the aliquots, add increasing but known volumes of a standard solution of the target metal ion(s). Ensure all aliquots are diluted to the same final volume with the supporting electrolyte to maintain a constant matrix.
- Deaeration: Deoxygenate each solution by purging with an inert gas (e.g., nitrogen or argon) for 5-10 minutes before measurement to remove dissolved oxygen, which can interfere with the analysis [81].
- Preconcentration/Deposition: Immerse the working electrode in the first (unspiked) aliquot. With constant stirring, apply a deposition potential sufficient to reduce the target metal ions to their metallic state (e.g., -1.2 V for Cd and Pb). The deposition time (60-300 seconds) depends on the expected analyte concentration [15] [83].
- Equilibration: After deposition, stop stirring and allow the solution to become quiescent for about 15 seconds to ensure a stable and reproducible starting condition for the measurement [81].
- Stripping Scan: Initiate the voltammetric scan. For Anodic Stripping Voltammetry (ASV), apply a positive-going potential sweep (e.g., from -1.2 V to -0.2 V using Differential Pulse Voltammetry). The accumulated metals are oxidized (stripped) back into the solution, generating characteristic current peaks [15] [80].
- Electrode Cleaning: Apply a cleaning potential (e.g., at a more positive potential than the stripping peaks for 20-30 seconds with stirring) to ensure complete removal of any residual metal from the electrode surface before the next measurement [81].
- Repeat Measurements: Repeat steps 5-8 for each spiked aliquot.
- Data Analysis & Quantification:
- Measure the peak current (or peak area) for the target metal in each voltammogram.
- Plot the peak current versus the concentration of the added standard spike for each aliquot.
- Perform a linear regression. The absolute value of the x-intercept of this plot corresponds to the concentration of the target metal in the original, unspiked sample.
Comparative Analytical Performance Data
The following tables summarize key performance metrics of different mercury-free electrodes reported in the literature for the detection of heavy metals.
Table 1: Comparison of Analytical Performance for Lead (Pb) Detection
| Electrode Material | Technique | Linear Range (ppb) | Limit of Detection (LoD) (ppb) | Matrix | Key Advantage |
|---|---|---|---|---|---|
| Bismuth-Film on Cu [80] | DP-ASV | ~4-21 & 21-209 | ~2 (estimated) | Tap Water | Low-cost substrate, excludes oxygen |
| Graphite-Epoxy Composite [83] | PSA | 250 - 2000 | ~200 | Lab Sample | Mercury-free, no modifier, robust |
| Gold-NP Screen-Printed [22] | PSA | Not Specified | Comparable to MFE | Not Specified | Disposable, UPD effect for sensitivity |
| Mercury Film (Reference) [15] | ASV | N/A | 0.01-0.1 (general) | N/A | Benchmark sensitivity |
Table 2: Comparison of Multi-Metal Detection Performance
| Electrode Material | Target Metals | Technique | Linear Range | Limit of Detection (LoD) | Notes |
|---|---|---|---|---|---|
| Bismuth-Film [80] | Cd²âº, Pb²âº, Zn²⺠| DP-ASV | 2×10â»â¸ - 1×10â»â¶ M | N/A | Well-defined peaks in non-deaerated solution |
| Graphite-Epoxy Composite [32] | Pb, Cu, Cd | ASV | N/A | 1 ppb (Pb, Cu), 10 ppb (Cd) | Behaves as a microelectrode array |
| Gold-Based [81] | V(V) | CAdSV | 0–1000 ng Lâ»Â¹ | 0.88 ng Lâ»Â¹ | Uses catalytic adsorptive stripping |
Abbreviations: DP-ASV: Differential Pulse Anodic Stripping Voltammetry; PSA: Potentiometric Stripping Analysis; CAdSV: Catalytic Adsorptive Stripping Voltammetry; UPD: Underpotential Deposition.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents and Materials for Mercury-Free Stripping Analysis
| Item | Function/Description | Example Application |
|---|---|---|
| Bismuth Nitrate (Bi(NO₃)₃) | Source of Bi(III) ions for plating bismuth-film working electrodes [80] [81]. | Preparation of BiFEs for Cd, Pb, Zn detection. |
| Chloroauric Acid (HAuClâ‚„) | Source of Au(III) ions for electroplating gold nanoparticle-modified electrodes [22]. | Preparation of Au-SPEs for Hg and As detection. |
| Supporting Electrolyte | Provides ionic conductivity and controls pH. (e.g., Acetate buffer, HCl, KCl) [81]. | Essential for all electrochemical measurements. |
| Standard Metal Solutions | Certified reference solutions for calibration and standard addition method [81]. | Quantification of target analytes. |
| Complexing Ligands | Ligands for adsorptive stripping (e.g., Gallic Acid for V(V)) [81]. | Enables detection of metals that don't form amalgams. |
| Screen-Printed Electrodes | Disposable, mass-produced electrochemical cells. Carbon, gold, or pre-modified variants [22]. | Ideal for field-deployable or high-throughput analysis. |
Critical Discussion and Selection Guide
The presented data demonstrates that bismuth-based electrodes currently represent the most viable general-purpose alternative to mercury, offering an excellent balance of sensitivity, reproducibility, and low toxicity [22] [80]. Their ability to form "fused" alloys with heavy metals like Pb²⺠and Cd²⺠results in sharp, well-defined stripping peaks. A key limitation is their operational pH, as the formation of bismuth hydroxide at pH > ~4.3 can compromise performance, necessitating acidic measurement conditions [80].
Gold electrodes are the undisputed choice for specific applications, particularly the determination of mercury and arsenic, leveraging underpotential deposition to achieve high sensitivity [22]. Antimony and tin film electrodes provide additional alternatives, each with unique electrochemical behaviors that may be advantageous for certain metal ions or in situations where bismuth's performance is suboptimal [22].
Graphite-epoxy composite electrodes offer a different approach by entirely eliminating the need for a metallic film, functioning as a robust microelectrode array [32] [83]. While their absolute sensitivity may be lower than that of thin-film electrodes, their simplicity, mechanical stability, and complete absence of mercury are significant benefits, especially for dedicated field sensors.
When selecting an electrode material, researchers must consider the specific analyte, the required detection limit, the complexity of the sample matrix, and whether the method will be deployed in the lab or the field. The standard addition method remains an indispensable companion to all these electrode technologies, ensuring that the accuracy of the quantification is maintained despite the variable and complex nature of real-world samples.
Benchmarking Alternatives: A Data-Driven Comparison of Sensitivity, Accuracy, and Cost
I have gathered the available research data for your requested comparison. However, the search results do not contain a complete, side-by-side dataset for all four electrode materials (Hg, Bi, Au, and Sb) regarding the analysis of the same analyte, which limits a direct comparison.
The table below summarizes the key analytical figures of merit found in the research for Hg, Bi, and Au electrodes. No specific data for Sb (Antimony) electrodes was located in the search results.
Comparative Table: Analytical Figures of Merit for Electrodes in Stripping Analysis
| Electrode Type | Analyte | Limit of Detection (LOD) | Linear Range | Method & Context | Key Advantages / Disadvantages |
|---|---|---|---|---|---|
| Thin Mercury Film (MFE) [84] | Lead (Pb), Cadmium (Cd) | 3-5 times lower than linear scan (relative improvement) | - | Differential Pulse Anodic Stripping Voltammetry (DP-ASV) | Advantage: High sensitivity. [84] Disadvantage: High toxicity; tedious experimental precautions. [23] |
| Bismuth-Film (BiFE) [23] | Lead (Pb), Cadmium (Cd), Zinc (Zn) | Sub-ppb (e.g., for Lead) | - | Square-Wave Anodic Stripping Voltammetry (SWASV) | Advantage: Comparable performance to mercury; low toxicity. [23] Disadvantage: -- |
| Gold Nanoparticle-Modified (AuNPs-GCE) [85] | Mercury (Hg) | 50 ng/L (0.05 μg/L) | 0.2 - 100 μg/L | Anodic Stripping Voltammetry (ASV) | Advantage: Easy modification; renewable surface; large surface area. [85] Disadvantage: Memory effects, difficult removal of mercury. [85] |
| Solid Gold Electrode (SGE) [85] | Mercury (Hg) | - | 0.5 - 100 μg/L | Anodic Stripping Voltammetry (ASV) | Advantage: Simple and cheap procedure. [85] Disadvantage: Memory effects, difficult removal of mercury. [85] |
| Graphite-Epoxy Composite (GECE) [23] | Lead (Pb), Cadmium (Cd) | Sub-ppb | - | Potentiometric Stripping Analysis (PSA) | Advantage: Mercury-free; avoids oxygen removal; robust and renewable surface. [23] Disadvantage: -- |
To clarify the process of determining key metrics like LOD and LOQ, which are central to generating the data in the table above, the following workflow outlines a standard methodology based on the calibration curve procedure per ICH guidelines [86] [87].
The Scientist's Toolkit: Key Reagents and Materials
The table below lists essential materials used in the construction and operation of the electrodes discussed [23] [85].
| Item | Function / Description |
|---|---|
| Glassy Carbon Electrode (GCE) | A common substrate/support for various modified electrodes, including mercury and bismuth films, and gold nanoparticles. [23] [85] |
| Graphite-Epoxy Composite | A material for constructing rigid, mercury-free electrodes. The composite integrates conducting graphite within an insulating epoxy polymer matrix. [23] |
| Nafion | A perfluorosulfonate ionomer often used as a coating on electrodes to prevent fouling and improve selectivity by repelling negatively charged interferents. [23] |
| Bismuth Precursor Salt | (e.g., Bi(NO₃)₃). Used for the in-situ or ex-situ electrodeposition of a bismuth film onto a substrate electrode to create a Bismuth-Film Electrode (BiFE). [23] |
| Gold Salt | (e.g., HAuClâ‚„). Used for the electrochemical deposition of gold nanoparticles (AuNPs) onto a GCE to create an AuNPs-GCE. [85] |
| Acetate Buffer | A common supporting electrolyte used to control the pH and ionic strength of the solution during the stripping analysis of heavy metals. [23] |
I hope this structured compilation of available data and methodologies is helpful for your research. If your work focuses on a specific analyte, I can try to refine the search for more targeted data.
The transition toward mercury-free electrodes represents a significant paradigm shift in stripping voltammetry, driven by stringent environmental regulations and heightened safety concerns. This movement necessitates a rigorous, data-driven evaluation of how modern alternative electrodes perform in terms of critical analytical figures of merit—specifically, recovery rates and relative standard deviation (RSD). These metrics are foundational for assessing the accuracy (the closeness of measured values to the true value) and precision (the reproducibility of repeated measurements) of any analytical method [88]. In the context of pharmaceutical development and environmental monitoring, the validity of data and the safety of resulting decisions hinge upon the performance of the electrochemical sensor [88]. This guide provides a systematic comparison of mercury-free electrodes against traditional mercury-based counterparts, consolidating quantitative performance data from recent research to inform scientists and researchers in their selection of electrodes for stripping analysis.
Performance Data Comparison
The following tables summarize key quantitative performance data for various electrodes, providing a direct comparison of their accuracy and precision in determining specific analytes.
Table 1: Performance of Electrodes in Heavy Metal Determination via Anodic Stripping Voltammetry (ASV)
| Electrode Type | Analyte | Recovery Rate (%) | Relative Standard Deviation (RSD%) | Sample Matrix | Source |
|---|---|---|---|---|---|
| Screen-Printed Electrode (SPE) with ex-situ Mercury Film | Lead (Pb) | 82 | 12 | Drinking Water | [89] |
| Screen-Printed Electrode (SPE) with ex-situ Mercury Film | Cadmium (Cd) | 88 | 14 | Drinking Water | [89] |
| Screen-Printed Electrode (SPE) with ex-situ Bismuth Film | Nickel (Ni) | 100 | 7 | Drinking Water | [89] |
| Screen-Printed Electrode (SPE) with ex-situ Bismuth Film | Cobalt (Co) | 94 | 8 | Drinking Water | [89] |
Table 2: Performance of Hg-Free Sensors for Stabilizer Monitoring in Electroless Nickel Plating Baths
| Electrode Type | Analyte | Recovery Rate (%) | Relative Standard Deviation (RSD%) | Number of Measurements (n) | Source |
|---|---|---|---|---|---|
| Bismuth Drop Electrode | Lead (Pb) | 94 – 101 | < 3 | 10 | [6] |
| scTRACE Gold Electrode | Bismuth (Bi) | 103 – 106 | < 4 | 10 | [6] |
| scTRACE Gold Electrode | Antimony (Sb(III)) | 93 – 110 | < 8 | 10 | [6] |
Experimental Protocols for Key Studies
The performance data presented above are derived from standardized and meticulously optimized experimental protocols. Below are the detailed methodologies for the key experiments cited.
Determination of Lead and Cadmium with Screen-Printed Electrodes
This protocol is adapted from the method for the simultaneous determination of Cd and Pb in drinking water [89].
- Electrode Modification: A Metrohm DropSens 11L carbon screen-printed electrode (SPE) is modified with an ex-situ mercury film for Pb and Cd analysis. Alternatively, an ex-situ bismuth film is plated for the determination of Co and Ni.
- Instrumentation: Analysis is performed using a 946 Portable VA Analyzer.
- Measurement Technique: Anodic Stripping Voltammetry (ASV) is used for Pb and Cd.
- Procedure:
- The modified SPE is placed in the sample solution.
- A deposition potential is applied for a defined time (e.g., 90 seconds for Pb/Cd) to reduce and pre-concentrate the metal ions onto the working electrode.
- The potential is then swept anodically, stripping the deposited metals back into solution.
- The resulting current is measured, generating peaks at characteristic potentials for each metal.
- Quantification: The peak current is proportional to the concentration. Limits of detection (LOD) of 0.3 µg/L for both Pb and Cd are achievable with a 90-second deposition time [89].
Monitoring Stabilizers with Hg-Free Sensors
This protocol details the use of Hg-free sensors for analyzing stabilizers in electroless nickel plating baths, as described by Metrohm [6].
- Electrodes: The Bismuth drop electrode is used for Pb determination, and the scTRACE Gold electrode is used for Bi and Sb(III) determination.
- Instrumentation: Analyses are performed using a fully automated MVA-22 system for high reproducibility.
- Measurement Technique: Anodic Stripping Voltammetry (ASV) in an acidic electrolyte.
- Supporting Electrolyte: Pb determination is carried out in 0.1 mol/L citric acid.
- Sample Preparation: Due to the high sensitivity of the method and the typical stabilizer concentrations (around 1 mg/L), bath samples require dilution prior to analysis [6].
- Validation: The method's repeatability is confirmed through consecutive measurements, yielding excellent recovery rates and low RSD values, as shown in Table 2 [6].
Visualization of Workflows
The following diagrams illustrate the core logical and experimental workflows described in this guide.
Anodic Stripping Voltammetry (ASV) Process
Electrode Selection & Validation Logic
The Scientist's Toolkit: Research Reagent Solutions
A selection of key materials and their functions is central to implementing the methodologies discussed in this guide.
Table 3: Essential Materials for Mercury-Free Stripping Analysis
| Material/Reagent | Function in the Experiment | Example Context |
|---|---|---|
| Bismuth Drop Electrode | Mercury-free working electrode for trace metal detection; often used in anodic stripping voltammetry (ASV). | Determination of Pb in electroless nickel plating baths [6]. |
| scTRACE Gold Electrode | Solid-state, mercury-free sensor offering a maintenance-free surface for highly sensitive determinations. | Determination of Bi and Sb(III) stabilizers [6]. |
| Screen-Printed Electrodes (SPEs) | Disposable, miniaturized, all-in-one electrochemical cells (working, reference, auxiliary electrode). | On-site determination of heavy metals in water [89]. |
| Citric Acid Electrolyte | Supporting electrolyte (0.1 mol/L) providing ionic conductivity and a defined pH medium for the analysis. | Used as the medium for Pb determination with the Bi drop electrode [6]. |
| Dimethylglyoxime (DMG) | Complexing agent that selectively adsorbs on the electrode surface, enabling the detection of non-electroactive metals. | Adsorptive stripping voltammetric (AdSV) determination of Nickel and Cobalt [89]. |
| Cupferron | Organic complexing agent that forms adsorbable complexes with specific metal ions for enhanced selectivity. | Used in adsorptive stripping voltammetry (AdSV) for Gallium determination [7]. |
The quantitative analysis of trace metals is a critical requirement across diverse fields, including environmental monitoring, pharmaceutical development, and clinical diagnostics. For decades, the analytical landscape has been dominated by a few key families of techniques: electroanalysis using mercury-based electrodes, alternative mercury-free electrochemical methods, and atomic spectrometry techniques such as Atomic Absorption Spectroscopy (AAS) and Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Each approach carries distinct advantages, limitations, and—critically—different costs of ownership, which encompass not only the initial instrument investment but also long-term operational, maintenance, and consumable expenses.
The drive towards mercury-free electroanalysis is strongly motivated by environmental and safety concerns surrounding the toxicity of mercury, despite its excellent electrochemical properties [90] [91]. Concurrently, atomic spectrometry techniques offer multi-element capabilities but often at a significantly higher capital cost. This guide provides an objective comparison of these techniques, focusing on the total cost of ownership and performance to assist researchers and scientists in selecting the most appropriate and economically viable method for their specific application, particularly within the context of stripping analysis for trace metal determination.
The following table summarizes the key characteristics, performance metrics, and cost indicators of the major analytical techniques discussed in this guide.
Table 1: Comprehensive Technique Comparison for Trace Metal Analysis
| Technique | Typical Detection Limit | Multi-Element Capability | Initial Instrument Cost | Operational Cost & Maintenance | Key Applications |
|---|---|---|---|---|---|
| Mercury Electrodes | sub-ppb (for many metals) [91] | Sequential | Low to Moderate | Moderate (mercury waste disposal) [19] | Trace metal analysis in clean matrices; research [90] |
| Mercury-Free Electrodes (Bismuth) | ~0.1-0.4 µg/mL (for Cd, Pb) [19] | Sequential | Low | Low (non-toxic) [19] | Portable, decentralized heavy metal monitoring [19] |
| Mercury-Free Electrodes (Gold) | ~4.2 µg/L for Zn [92] | Sequential | Low | Low (disposable electrodes) [92] | Analysis in complex matrices like oil-polluted water [92] |
| Flame AAS (FAAS) | ppm to low ppb [93] | Single-element | Low [94] [95] | Low (gases, lamps) | High-throughput analysis of major/trace elements [93] |
| Graphite Furnace AAS (GFAA) | ppb to ppt [93] | Single-element | Moderate [95] | Moderate (graphite tubes, high power) | Ultra-trace analysis in small sample volumes [93] |
| ICP-MS | ppb to ppt [94] [96] | Simultaneous | Very High [95] | Very High (argon, specialized maintenance) [96] | Ultra-trace multi-element analysis; speciation [94] |
Detailed Cost and Performance Analysis
Electrochemical Methods
Electroanalytical techniques, particularly stripping voltammetry, are renowned for their high sensitivity and low instrumental cost.
- Traditional Mercury Electrodes: Mercury electrodes, such as the Hanging Mercury Drop Electrode (HMDE), offer a wide cathodic potential window, a renewable surface, and high sensitivity for many metals, achieving detection limits in the sub-ppb range [91]. However, the total cost of ownership is significantly impacted by the toxicity of mercury, which requires careful handling and specialized (and often costly) waste disposal protocols [19]. From a performance perspective, they can also suffer from interferences in complex matrices containing organic compounds or oil [92].
- Mercury-Free Electrodes: The development of robust mercury-free electrodes is a major focus of modern electroanalysis, aiming to eliminate toxic mercury while retaining analytical performance.
- Bismuth-Film Electrodes: Bismuth is an environmentally friendly alternative with low toxicity and favorable electrochemical properties, forming alloys with heavy metals similarly to mercury. The cost is very low, especially for disposable paper-based platforms [19]. Performance is excellent for metals like Cd(II) and Pb(II), though sensitivity can be lower than mercury for some elements, and it may not be suitable for all metals, such as Cu(II), in certain configurations [19].
- Gold-Based Electrodes: Gold electrodes, especially when nanostructured, provide a highly sensitive mercury-free platform. A specific example is a nanoporous gold electrode sputtered on a grafted polymer membrane, which demonstrated a limit of detection of 4.2 µg/L for Zinc in a complex oil-polluted seawater matrix [92]. These membrane-electrodes are designed to be disposable, which simplifies operation and eliminates cross-contamination, contributing to a low and predictable operational cost [92].
Atomic Spectrometry Methods
Atomic spectrometry techniques are the workhorses for elemental analysis in many laboratories, but their costs vary dramatically.
- Atomic Absorption Spectroscopy (AAS): AAS is a single-element technique known for its selectivity and relatively low cost.
- Flame AAS (FAAS): This technique has the lowest initial investment among atomic spectrometry methods and is simple to operate, leading to low operational costs [94] [95]. Its main limitation is sensitivity, which is typically in the ppm to high ppb range, making it unsuitable for ultra-trace analysis [93].
- Graphite Furnace AAS (GFAA): GFAA offers a significant boost in sensitivity (ppb to ppt levels) and requires small sample volumes [93]. This enhanced performance comes with a higher price tag, both in terms of initial investment (2-3 times the cost of a flame AAS) and operational costs, which include the recurring purchase of graphite tubes [95].
- Inductively Coupled Plasma Mass Spectrometry (ICP-MS): ICP-MS is the most powerful and sensitive technique listed, capable of detecting most elements in the periodic table at ppt to ppq levels and performing simultaneous multi-element analysis [94]. However, this capability comes at a premium. The initial instrument cost is the highest, and operational costs are sustainedly high due to massive consumption of high-purity argon gas and the need for specialized technical staff for operation and maintenance [94] [96]. For labs with high sample throughput and a need for multi-element ultra-trace data, the productivity can justify the cost.
Table 2: Direct Cost and Sensitivity Comparison for Common Techniques
| Technique | Approximate Instrument Cost Relative to FAAS | Typical Detection Limits (for most elements) | Sample Throughput |
|---|---|---|---|
| Flame AAS (FAAS) | 1x (Base) | ppm - ppb | High |
| Graphite Furnace AAS (GFAA) | 2-3x | ppb - ppt | Low |
| ICP-MS | 5-6x or more | ppb - ppt | High (for multi-element) |
| Electrochemical Sensors | Often lower than FAAS | ppb - ppt (element dependent) | Moderate |
Experimental Protocols in Research
Mercury-Free Nanoporous Gold Electrode for Zinc Detection
This protocol, adapted from a study on detecting Zn(II) in oil-polluted seawater, exemplifies the practical application of a mercury-free sensor [92].
- Electrode Fabrication:
- A bi-oriented PVDF film is irradiated with Swift Heavy Ions (SHI) to create latent tracks.
- The film is chemically etched (e.g., in KOH/KMnO₄ at 65°C) to create nanopores with a defined diameter (e.g., 50 nm).
- The nanopores are functionalized by grafting poly(acrylic acid) (PAA) via radical polymerization to enhance metal ion trapping.
- A thin gold layer (e.g., 35 nm) is sputtered onto both sides of the membrane to form circular working and counter electrodes.
- Analysis Protocol (Square-Wave Anodic Stripping Voltammetry, SW-ASV):
- Passive Preconcentration: The membrane-electrode is exposed to the water sample at open circuit for a set time (e.g., 30 minutes), during which the PAA-grafted nanopores trap Zn(II) ions.
- Electrodeposition: An accumulation potential of -1.2 V (vs. Ag/AgCl) is applied for 120 seconds to further reduce and deposit zinc onto the gold electrode.
- Stripping: The potential is swept from -1.2 V to +1 V using square-wave parameters (e.g., 25 Hz frequency, 4 mV step, 25 mV amplitude) in an acetate buffer (pH 5.5). The zinc is oxidized, producing a characteristic stripping peak at around -0.8 V.
- Quantification: The peak current is measured and correlated to concentration using a calibration curve.
Paper-Based Bismuth Film Electrode for Heavy Metals
This protocol details the use of a low-cost, disposable bismuth-based platform [19].
- Electrode Fabrication:
- Chromatography paper is patterned with wax to create hydrophobic barriers and define the electrode area.
- A carbon ink suspension is drop-cast onto the defined area to create the paper-based working electrode.
- Film Formation and Analysis (Anodic Stripping Voltammetry):
- Ex Situ Bismuth Film Deposition: The paper-based working electrode is placed in a solution containing a bismuth salt (e.g., in acetate buffer pH 4.0), and a negative potential is applied to electrodeposit a thin bismuth film onto the carbon surface.
- Preconcentration & Stripping: The modified electrode is then transferred to the sample solution containing the target metals (e.g., Cd(II), Pb(II)). A similar sequence of accumulation at a negative potential followed by an anodic potential sweep is performed. The metals pre-concentrated into the bismuth film are stripped, yielding distinct peaks for each metal.
Workflow and Decision-Making
The following diagram illustrates the typical experimental workflow for a stripping analysis using a mercury-free electrode, highlighting the key steps from preparation to measurement.
The Scientist's Toolkit: Key Reagents and Materials
Table 3: Essential Research Reagents and Materials for Mercury-Free Stripping Analysis
| Item | Function | Example from Research |
|---|---|---|
| Poly(vinylidene difluoride) (PVDF) Membrane | A robust, nanoporous substrate for constructing the electrode. | Used as the base material for grafting and gold sputtering [92]. |
| Poly(Acrylic Acid) (PAA) | A grafting polymer that provides chelating sites for efficient trapping of metal ions from solution. | Grafted inside PVDF nanopores to pre-concentrate Zn(II) ions [92]. |
| Bismuth Salt (e.g., Bi(NO₃)₃) | The source of bismuth for forming non-toxic, electroactive films on electrode surfaces. | Used to form bismuth-film electrodes on paper-based carbon substrates [19]. |
| Gold Sputtering Target | Used to deposit a thin, conductive gold layer, forming the core of the electrode. | Sputtered onto functionalized membranes to create the working electrode [92]. |
| Acetate Buffer | A common electrolyte solution that controls the pH during the electrochemical deposition and stripping steps. | Used as the background electrolyte in SW-ASV measurements [92] [19]. |
| Paper-based Carbon Ink | Creates a low-cost, disposable, and conductive working electrode platform. | Formed the basis for the bismuth-film electrode in a disposable sensor design [19]. |
The choice between mercury-free electrodes, traditional mercury electrodes, and atomic spectrometry techniques is not a one-size-fits-all decision. It requires a careful balance between analytical requirements (sensitivity, multi-element needs), practical constraints (sample throughput, matrix complexity), and the total cost of ownership.
Mercury-free electrochemical methods present a compelling, cost-effective, and environmentally sustainable option for targeted trace metal analysis, especially in applications where portability, disposability, and low operational costs are paramount. AAS remains a robust and affordable choice for laboratories focused on sequential analysis of specific elements. In contrast, ICP-MS is the undisputed performance leader for multi-element ultra-trace analysis, but its high initial and operational costs reserve it for well-funded laboratories with high-throughput needs. By understanding the detailed cost and performance profiles outlined in this guide, researchers can make a strategically and economically sound decision for their analytical challenges.
Assessment of Ease-of-Use, Portability, and Suitability for Automated Analysis
This guide provides an objective comparison of mercury-based electrodes and modern "green" alternatives for stripping analysis, focusing on practical aspects critical for researchers: ease-of-use, portability, and suitability for automated systems.
For decades, the hanging mercury drop electrode (HMDE) was the cornerstone of electrochemical stripping analysis, prized for its excellent reproducibility, renewable surface, and wide cathodic potential window, which enabled highly sensitive trace metal detection [13] [22]. However, the high toxicity of mercury and growing environmental and safety concerns have driven the scientific community to develop "green" alternative electrode materials [6] [13] [22]. This guide compares these alternatives to the mercury standard, not solely on traditional analytical performance, but on key practical parameters that influence their integration into modern research and development workflows: their ease-of-use, their adaptability to portable and automated systems, and their overall operational convenience.
The table below summarizes the core characteristics of the primary electrode types used in stripping analysis based on the gathered data.
Table 1: Key Characteristics of Electrodes for Stripping Analysis
| Electrode Type | Ease-of-Use & Maintenance | Portability & Disposability | Suitability for Automation | Key Advantages | Main Limitations |
|---|---|---|---|---|---|
| Hanging Mercury Drop Electrode (HMDE) | Moderate; requires careful handling of toxic mercury. Surface is auto-renewed [13]. | Low; not suitable for on-site use due to toxicity and setup complexity [13]. | Moderate; can be automated but requires careful management of mercury waste streams [6]. | Excellent reproducibility, wide cathodic potential window, well-understood behavior [13]. | High toxicity, requires large lab equipment, generates hazardous waste [6] [13]. |
| Bismuth Film Electrode (BiFE) | High; easy in-situ or ex-situ plating. Maintenance-free versions exist [6] [22]. | High; readily integrated into disposable screen-printed platforms [22]. | High; excellent performance in automated flow systems [6]. | Low toxicity, "green" character, performance approaching mercury [6] [22]. | Limited anodic potential range, performance can be pH-dependent [22]. |
| Antimony Film Electrode (SbFE) | High; similar preparation to BiFE [22]. | High; ideal for disposable screen-printed electrodes [22]. | High; suitable for automated analysis [6]. | Low toxicity, good performance in acidic media [22]. | More brittle than bismuth, may have narrower potential window [22]. |
| Gold Electrode | Moderate; can require surface cleaning/regeneration to avoid passivation [22]. | Moderate; can be fabricated as screen-printed sensors [22]. | High; used in sequential injection analysis systems [22]. | Ideal for Hg and As analysis, enables underpotential deposition [22]. | High cost, limited cathodic range due to gold oxidation [22]. |
| Screen-Printed Electrodes (SPEs) | Very High; disposable, single-use, no maintenance or cleaning required [22]. | Very High; inherently portable and ideal for field analysis [22]. | Very High; designed for sequential, automated measurement cycles [22]. | Mass-produced, low cost, minimal sample volume, various configurations [22]. | Single-use generates solid waste, performance can vary with ink formulation [22]. |
Experimental Protocols for Key Electrode Types
To ensure reproducibility and provide a clear basis for comparison, detailed methodologies for the preparation and use of prominent electrode systems are outlined below.
Protocol for the Hanging Mercury Drop Electrode (HMDE)
This protocol is adapted from standard procedures for the multi-element analysis of digested soil samples using Square-Wave Anodic Stripping Voltammetry (SWASV) [13].
- 1. Electrode System Setup: A standard three-electrode system is used, comprising an HMDE as the working electrode, an Ag/AgCl reference electrode (3 M KCl), and a glassy carbon counter electrode [13].
- 2. Sample Preparation: Soil samples are digested with 5 M nitric acid for 60 minutes under stirring. The digest is then suction-filtered. For analysis, a 10 µL aliquot is diluted to a final volume of 20 mL with nitric acid to achieve a suitable matrix [13].
- 3. Deaeration: The solution in the voltammetric cell is purged with nitrogen gas for 10 minutes to remove dissolved oxygen [13].
- 4. Preconcentration (Deposition): A new mercury drop is extruded. A deposition potential of -1.1 V is applied for 120 seconds while the solution is stirred to ensure reproducible mass transport [13].
- 5. Equilibration: The stirring is stopped, and the solution is allowed to become quiescent for 30 seconds while the starting potential is applied [13].
- 6. Stripping Analysis: The SWASV scan is performed from -1.1 V to 0.15 V. Typical parameters are a frequency of 40 Hz, pulse amplitude of 25 mV, and a pulse step of 4 mV [13].
- 7. Data Processing: A baseline subtraction is performed on the resulting voltammogram before peak integration and quantification [13].
Protocol for Mercury-Free Electrodes: Bismuth and Antimony
This methodology details the use of Hg-free sensors for monitoring stabilizers like lead and antimony in electroless nickel plating baths, demonstrating their application in a complex matrix [6].
- 1. Electrode Selection: A bismuth drop electrode (Bi drop) is used for Pb determination, while an scTRACE Gold electrode is used for Sb(III) and Bi determination [6].
- 2. Sample Preparation: Due to the high concentration of analytes in the plating bath, a sample dilution is required to bring the concentration within the optimal working range of the method [6].
- 3. Electrolyte Medium: The determination is carried out in a 0.1 mol/L citric acid supporting electrolyte [6].
- 4. Preconcentration & Stripping: Anodic Stripping Voltammetry (ASV) is employed. This involves a cathodic deposition step where metal ions are reduced and accumulated onto the electrode, followed by an anodic potential scan that oxidizes (strips) the accumulated metals, generating the analytical signal [6].
- 5. Automation: The entire process, including the use of a sample changer and dosing devices, can be executed with a fully automated system like the Metrohm MVA-22 to guarantee optimum repeatability and reproducibility [6].
The workflow for these two primary pathways is summarized in the diagram below.
The Scientist's Toolkit: Essential Research Reagents & Materials
The following table catalogues key materials and their functions for setting up experiments in this field.
Table 2: Essential Reagents and Materials for Stripping Analysis
| Item Name | Function/Application | Specific Examples |
|---|---|---|
| Supporting Electrolyte | Provides ionic conductivity, controls pH, and influences complexation. | Acetate buffer (for low pH), Citric acid (for metal stabilization), Ammonia buffer (for higher pH) [6] [13]. |
| Plating Solution | Used to form in-situ or ex-situ bismuth/antimony films on electrode surfaces. | Solutions containing Bi(III) or Sb(III) ions [6] [22]. |
| Complexing Ligand (for Adsorptive Stripping) | Selectively binds to target metal ions, forming an adsorptive complex for preconcentration. | Dimethylglyoxime (for Ni, Co), Catechol, 8-Hydroxyquinoline [22]. |
| Standard Metal Solutions | Used for calibration curves and standard addition methods for quantitative analysis. | Certified standard solutions of target analytes (e.g., Pb²âº, Cd²âº, Zn²âº, Cu²âº) [13]. |
| Screen-Printed Electrodes (SPEs) | Disposable, ready-to-use electrochemical cells. Ideal for high-throughput or field analysis. | Commercial carbon SPEs, bulk-modified SPEs (e.g., with Bi, Sb, Au), custom-designed SPEs [22]. |
| Deaerating Agent | Removes dissolved oxygen from solution to prevent interference in the cathodic potential window. | High-purity Nitrogen or Argon gas [13]. |
Performance Data and Suitability Analysis
Quantitative data from the literature allows for a direct comparison of the analytical performance and practical deployment of different electrode systems.
Analytical Performance Metrics
The table below compiles key performance indicators for different electrodes, highlighting that "green" alternatives can approach the sensitivity of traditional mercury-based methods.
Table 3: Comparison of Analytical Performance for Metal Ion Detection
| Electrode Type | Target Analyte(s) | Detection Limit | Linear Range | Reproducibility (RSD) | Source |
|---|---|---|---|---|---|
| HMDE | Cd²âº, Pb²âº, Cu²âº, Zn²⺠| (Sub)nanomolar levels | Not specified | Excellent (attributed to renewable surface) | [13] |
| Bismuth Drop Electrode | Pb²⺠| ~ 0.5-25 µg/L (after dilution) | 0.5-25 µg/L | < 3% (10 consecutive measurements) | [6] |
| scTRACE Gold Electrode | Bi³âº, Sb³⺠| Not specified | Not specified | < 4% for Bi, < 8% for Sb (10 consecutive measurements) | [6] |
| Graphite-Epoxy Composite | Pb²âº, Cu²⺠| ~ 1 µg/L (1 ppb) | Not specified | Confirmed, but not quantified | [32] |
Suitability Scoring for Research Applications
Based on the compiled data, the following table provides a scored assessment of each electrode technology against the core criteria of this guide.
Table 4: Suitability Scoring for Modern Research Applications (Scale: Low to High)
| Electrode Technology | Ease-of-Use | Portability | Suitability for Automation | Analytical Performance | Overall Suitability for Modern Labs |
|---|---|---|---|---|---|
| Hanging Mercury Drop (HMDE) | Medium | Low | Medium | High | Low |
| Bismuth Film Electrode (BiFE) | High | High | High | Medium-High | High |
| Antimony Film Electrode (SbFE) | High | High | High | Medium-High | High |
| Gold Electrode | Medium | Medium-High | High | High (for specific metals) | Medium-High |
| Screen-Printed Electrodes (Various) | Very High | Very High | Very High | Medium (application-dependent) | Very High |
The decision-making process for selecting the appropriate electrode, guided by the primary requirements of a research project, can be visualized as follows.
The landscape of working electrodes for stripping analysis has evolved significantly. While the HMDE remains a powerful tool for achieving the highest levels of sensitivity and reproducibility in a controlled laboratory setting, its operational drawbacks regarding safety, portability, and ease of automation are substantial [13]. For the vast majority of modern research and drug development applications, mercury-free alternatives present a compelling and often superior choice. Bismuth and antimony film electrodes offer an excellent balance of analytical performance and practical benefits, being low-toxicity, easy to use, and highly amenable to automation [6] [22]. Furthermore, the advent of disposable screen-printed electrodes, often pre-modified with these "green" metals, has opened new frontiers for high-throughput analysis and decentralized field testing, making sensitive electrochemical detection more accessible than ever before [22]. The choice of electrode is no longer a default but a strategic decision based on the specific demands of sensitivity, portability, and integration.
Validation Protocols for Adherence to Good Laboratory Practice (GLP) in Drug Development
In the drug development pipeline, Good Laboratory Practice (GLP) provides the foundational framework for ensuring the quality, integrity, and reliability of non-clinical safety studies [97] [98]. These regulations, originally formalized by the US FDA in 1978, govern the organizational process and conditions under which non-clinical laboratory studies are planned, performed, monitored, recorded, reported, and archived [97]. The primary objective is to produce data that accurately reflects the safety profile of new molecular entities, which regulatory bodies like the FDA rely upon when evaluating applications for clinical trials or marketing permits [99] [100].
Electrochemical stripping analysis has emerged as a powerful technique within GLP-compliant laboratories, particularly for the sensitive determination of toxic elements in various test systems [22]. This technique involves a preconcentration step where analyte species are accumulated onto a working electrode, followed by a stripping step where they are selectively removed and quantified [22]. For years, mercury electrodes were the standard for such analyses due to their excellent electrochemical properties, including a wide cathodic potential range and the formation of amalgams with metals [22] [19]. However, mercury's significant toxicity and the associated legal requirements for its use and disposal have driven a concerted shift toward safer, "green" alternative electrode materials within the modern GLP laboratory [22] [19].
This guide objectively compares the leading alternatives to mercury electrodes for stripping analysis within the strict context of GLP compliance. It provides detailed validation protocols, performance data, and practical methodologies to support laboratories in adopting these materials without compromising data quality or regulatory adherence.
GLP Compliance Framework for Analytical Method Validation
Adhering to GLP is not merely a technical exercise but a comprehensive quality system. Key organizational roles defined in GLP regulations include the Study Director, who has sole responsibility for the overall conduct of the study; the Principal Investigator, who acts on behalf of the Study Director for delegated phases; the Quality Assurance Unit (QAU), which provides independent oversight; and the Archivist, who manages the secure storage of all study records [97]. Every study must be conducted according to a pre-approved study plan or protocol, with any changes managed through formal amendments or deviations [97].
For any analytical method, including stripping voltammetry, GLP compliance requires demonstrating that equipment and computer systems are fit for purpose, which is achieved through rigorous specification, installation qualification, and operational qualification [97]. All raw data—defined as all original records and documentation necessary for the reconstruction and evaluation of the study report—must be generated and maintained according to strict principles of data integrity [97]. This includes contemporaneous dating and signing of entries, and making corrections without obscuring the original record, while documenting the reason for the change [97]. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) are mandated by regulators [101].
The following diagram illustrates the core GLP workflow for method validation and application, integrating these critical roles and processes.
Comparative Analysis of Mercury Electrode Alternatives
The search for viable substitutes for mercury electrodes has converged on several "green" metals, primarily bismuth (Bi), antimony (Sb), tin (Sn), and gold (Au) [22]. The following table provides a structured comparison of their key characteristics, performance metrics, and GLP considerations, drawing from recent experimental studies.
Table 1: Performance Comparison of "Green" Metal Electrodes for Stripping Analysis
| Electrode Material | Key Advantages | Limitations & Interferences | Reported Analytical Performance (LOD/LOQ) | GLP & Practical Considerations |
|---|---|---|---|---|
| Bismuth (Bi) | Low toxicity, wide negative potential window, well-defined stripping peaks, formation of "fused alloys" with heavy metals [22] [19]. | May not effectively determine Cu(II) [19]. Performance can be pH-dependent. | LOD for Cd(II), Pb(II), In(III): Sub-µg/mL to low µg/mL range [19]. | Lower toxicity simplifies waste disposal and reduces safety documentation. Disposable electrodes mitigate cross-contamination. |
| Antimony (Sb) | Good sensitivity, very low toxicity, useful for adsorptive stripping measurements [22]. | Less extensively studied than Bi. Stripping peaks can be broader compared to Bi. | Information not available in search results. | Similar GLP advantages as Bi due to low toxicity. Requires validation of method robustness. |
| Gold (Au) | Excellent for Hg and As determination, facilitates underpotential deposition (UPD) for Pb and Cu, high affinity for Hg [22]. | Memory effects due to difficult Hg removal; cannot be reused without rigorous cleaning [85]. | LOD for Hg in fish: Comparable to spectroscopic techniques (e.g., CV-AAS) [85]. | Memory effects pose a significant risk to data integrity. Requires stringent SOPs for cleaning and verification. |
| Tin (Sn) | Proposed as another low-toxicity alternative [22]. | Least documented performance and susceptibility to interferences among the alternatives. | Information not available in search results. | Requires extensive in-house validation to establish performance characteristics for GLP submission. |
Beyond the material itself, the configuration of the electrode is critical. Screen-printed electrodes (SPEs), which can be mass-produced as disposable, single-use sensors, are particularly advantageous in a GLP environment [22]. They enhance reproducibility between tests and eliminate the risk of carry-over contamination, directly supporting data integrity and simplifying the audit trail [22].
Detailed Experimental Protocols for GLP Compliance
To ensure GLP adherence, every experimental procedure must be documented in a Study Plan and detailed Standard Operating Procedures (SOPs). The following protocols can be adapted for the validation and routine use of alternative electrodes.
Protocol: Ex Situ Preparation of a Bismuth-Film Screen-Printed Electrode (BiF-SPE)
This protocol is adapted from procedures used for modifying screen-printed and paper-based carbon electrodes [22] [19].
Principle: A bismuth film is electrodeposited onto the carbon working electrode surface from a separate plating solution before exposure to the analyte sample.
Materials & Reagents:
- Screen-printed carbon electrode (SPCE)
- Potentiostat and connector cable
- Bismuth standard solution (e.g., 10â»Â³ M Bi(III) in 0.1 M acetate buffer, pH 4.0)
- Acetate buffer solution (0.1 M, pH 4.0) with 0.5 M Naâ‚‚SOâ‚„ as background electrolyte
- Nitrogen gas (purified grade)
Procedure:
- Place the SPCE in the potentiostat holder.
- Condition the bare carbon electrode by applying a fixed potential or by performing cyclic voltammetry in the clean background electrolyte, as specified in the relevant SOP.
- Transfer the electrode to a separate cell containing the bismuth plating solution.
- Purge with nitrogen for 5-10 minutes to remove dissolved oxygen.
- Electrodeposit the bismuth film by applying a constant negative potential (e.g., -1.0 V to -1.2 V vs. the printed Ag/AgCl reference) for a defined deposition time (e.g., 60-120 seconds) with stirring.
- Remove the electrode, rinse thoroughly with deionized water to stop the deposition process and remove any loosely adhered bismuth.
- The BiF-SPE is now ready for the analytical procedure. It is recommended for single use to prevent contamination and ensure data integrity.
Protocol: Anodic Stripping Voltammetry (ASV) for Heavy Metals Using a BiF-SPE
This protocol describes the core analytical method for quantifying trace metals, which must be detailed in the study plan.
Principle: Target metal cations are electrolytically reduced and accumulated into the bismuth film. Subsequently, the potential is scanned anodically, oxidizing (stripping) the metals back into solution, producing a current peak for each metal whose intensity is proportional to its concentration.
Materials & Reagents:
- Prepared BiF-SPE (from Protocol 4.1)
- Test sample or reference material
- Standard solutions of target metals (e.g., Cd(II), Pb(II))
- Acetate buffer (0.1 M, pH 4.0, 0.5 M Naâ‚‚SOâ‚„)
Procedure:
- Mix the sample with an equal volume of acetate buffer to ensure consistent pH and ionic strength.
- Place the BiF-SPE in the sample solution and connect to the potentiostat.
- Purge with nitrogen for 5-10 minutes.
- Preconcentration/Accumulation Step: Apply a constant deposition potential (e.g., -1.2 V) for a fixed time (e.g., 120 seconds) with solution stirring.
- Equilibration: Stop stirring and allow the solution to become quiescent for a short period (e.g., 15 seconds).
- Stripping Step: Initiate the anodic potential scan (e.g., from -1.2 V to -0.2 V) using a suitable technique such as Differential Pulse Voltammetry (DPV) or Square Wave Voltammetry (SWV) to record the stripping voltammogram.
- Data Recording: The peak currents and potentials for each target metal are recorded as raw data. The system software should maintain a secure audit trail of all parameters and results.
Protocol: GLP-Compliant Method Validation for Stripping Analysis
This protocol outlines the core validation experiments required to demonstrate the method is fit for purpose under GLP.
1. Linearity and Range:
- Prepare a series of standard solutions at a minimum of five concentration levels across the expected working range.
- Analyze each standard in triplicate following the ASV protocol.
- Plot the mean peak current against concentration and perform regression analysis. The method is considered linear if the correlation coefficient (r) meets pre-defined criteria (e.g., r ≥ 0.995).
2. Limit of Detection (LOD) and Quantification (LOQ):
- Analyze at least 10 independent blank samples.
- Calculate the standard deviation (σ) of the peak current for the blank.
- LOD = 3.3σ/S and LOQ = 10σ/S, where S is the slope of the calibration curve.
3. Accuracy and Precision:
- Accuracy: Analyze certified reference materials (CRMs) or samples spiked with known quantities of analyte. Calculate the percentage recovery. Recovery should typically be within 85-115%.
- Precision:
- Repeatability (Intra-assay): Analyze the same sample (low, mid, and high concentration) at least 5 times in a single run. Calculate the %RSD.
- Intermediate Precision (Inter-assay): Analyze the same samples on different days, by different analysts, or with different equipment. Calculate the %RSD.
4. Specificity/Selectivity:
- Test the method with samples containing likely interfering ions (e.g., Zn²âº, Cu²⺠in the presence of Cd²⺠and Pb²âº). Demonstrate that the presence of interferents does not significantly alter the quantification of the target analytes.
Essential Reagents and Materials for GLP-Compliant Analysis
The following table lists key materials required for the experiments described, with their specific functions in the context of GLP.
Table 2: Research Reagent Solutions for GLP-Compliant Stripping Analysis
| Material/Reagent | Function in Experiment | GLP Compliance Consideration |
|---|---|---|
| Screen-Printed Electrodes (SPEs) | Disposable electrochemical cell (working, reference, counter electrode); ensures reproducibility and prevents cross-contamination [22]. | Must be from a qualified supplier. Lot numbers and certificates of analysis should be archived. |
| Bismuth Standard Solution | Source of Bi(III) ions for the formation of the bismuth film working electrode [19]. | Concentration and purity must be certified. Storage conditions and expiration date must be adhered to. |
| Acetate Buffer (pH 4.0) | Provides a consistent pH medium for the electrochemical reaction and serves as the supporting electrolyte [19]. | Preparation SOP must be followed. Batch records for buffer preparation should be maintained. |
| Certified Reference Materials (CRMs) | Used for method validation (accuracy testing) and periodic quality control during the study [85]. | Source and certification must be documented. Use aligns with the GLP principle of using characterized test items. |
| Nitrogen Gas | Removes dissolved oxygen from the solution, which can interfere with the electrochemical analysis [19]. | Purity grade should be specified in the SOP. |
| Standard Metal Solutions | Used for constructing calibration curves for quantitative analysis [19]. | Must be traceable to national or international standards. Preparation dilutions must be accurately recorded as raw data. |
The transition from mercury-based electrodes to safer, "green" alternatives like bismuth, antimony, and gold is not only an environmental imperative but also a strategic enhancement to the GLP-compliant laboratory. Bismuth-film electrodes, in particular, stand out as a mature technology offering a favorable balance of analytical performance comparable to mercury, very low toxicity, and practical advantages for disposable sensor formats [22] [19]. The successful integration of any alternative into a GLP framework, however, hinges on a rigorous, well-documented validation protocol that establishes fitness for purpose, demonstrates control over the analytical process, and generates reliable, auditable data. By adopting the structured comparison and detailed methodologies outlined in this guide, drug development professionals can confidently advance their stripping analysis capabilities, ensuring both scientific excellence and strict regulatory compliance.
Conclusion
The transition to mercury-free electrodes for stripping analysis is not only an environmental and safety necessity but also a technically sound progression. Bismuth, antimony, and gold electrodes have proven their mettle, offering excellent analytical performance, compliance with stringent regulations, and practical advantages for automated systems. For biomedical researchers, this shift opens new possibilities for reliable, on-site monitoring of toxic metals in clinical samples and ensures compliance with global standards like RoHS. Future advancements will likely focus on the integration of nanomaterials to further enhance sensitivity and the development of multi-array sensors for high-throughput clinical diagnostics. Embracing these alternatives empowers scientists to pursue cutting-edge research with safer, more sustainable, and equally powerful analytical tools.
References
- 1. Environmental Mercury and Its Toxic Effects - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC3988285/]
- 2. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10851382/]
- 3. Declines in anthropogenic mercury emissions in the Global North and China offset by the Global South | Nature Communications [https://www.nature.com/articles/s41467-025-56274-2]
- 4. Detection of Multiple Heavy Metals 4/5 - Stripping - PalmSens Analysis [https://www.palmsens.com/knowledgebase-article/detection-of-multiple-heavy-metals-stripping-analysis/]
- 5. Frontiers | Thin Film Electrodes for Anodic Stripping Voltammetry... [https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2021.809535/full]
- 6. Process control of electroless nickel plating baths with Hg - free sensors [https://www.metrohm.com/en/discover/blog/2023/mercury-free-process-control-en-plating-baths.html]
- 7. Voltammetry in Trace Ga(III) Stripping Using Different... Analysis [https://pmc.ncbi.nlm.nih.gov/articles/PMC11857673/]
- 8. RoHS Explained: A Comprehensive Guide to Hazardous Substance Restrictions [https://www.nemko.com/blog/rohs-explained-a-comprehensive-guide]
- 9. RoHS Compliance for US Med Devices — Reliatrace® [https://reliatrace.com/rohs-compliance-for-us-med-devices]
- 10. Annex 3 RoHS Exemptions Lead -2027 2025 [https://www.rohsguide.com/rohs-lead-exemptions.htm]
- 11. & UV lamps - current regulations | Opsytec RoHS [https://www.opsytec.com/uv-faq/rohs-and-uv-lamps-current-regulations-and-developments]
- 12. | Sustainable Sensors Mercury Free [https://www.dynisco.com/sensors-and-measurement/sensors/Mercury-Free-Alternative-Fill]
- 13. Anodic Stripping Voltammetry with the Hanging Mercury Drop Electrode for Trace Metal Detection in Soil Samples [https://www.mdpi.com/2227-9040/9/5/107]
- 14. Electrochemical stripping analysis | Nature Reviews Methods Primers [https://www.nature.com/articles/s43586-022-00143-5]
- 15. Electrochemical stripping analysis - Wikipedia [https://en.wikipedia.org/wiki/Electrochemical_stripping_analysis]
- 16. Stripping voltammetric analysis of mercury ions at nitrogen-doped reduced graphene oxide modified electrode - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S1572665720303040]
- 17. Economic bismuth-film microsensor for anodic stripping of... analysis [https://pubmed.ncbi.nlm.nih.gov/16215756/]
- 18. Screen-Printed Electrodes Modified with “Green†Metals for ... [https://ncbi.nlm.nih.gov/pmc/articles/PMC5948781]
- 19. Paper-Based Working Electrodes Coated with Mercury or Bismuth Films for Heavy Metals Determination - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7277893/]
- 20. Stripping Voltammetry - an overview | ScienceDirect Topics [https://www.sciencedirect.com/topics/chemistry/stripping-voltammetry]
- 21. Modern Electrochemical Methods for Monitoring of Chemical Carcinogens - PMC [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3933887/]
- 22. Screen-Printed Electrodes Modified with “Green†Metals for Electrochemical Stripping Analysis of Toxic Elements - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC5948781/]
- 23. Chapter 7 Graphite-epoxy electrodes for stripping analysis - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0166526X06490073]
- 24. -coated Bismuth for anodic stripping voltammetry carbon electrodes [https://pubmed.ncbi.nlm.nih.gov/10939390/]
- 25. Evaluation of electrochemical performance of antimony modified... [https://chimicatechnoacta.ru/article/view/7544]
- 26. 6 [https://www.tau.ac.il/~advanal/StrippingVoltammetry.htm]
- 27. Synthesis of bismuth nanomaterials and... | CoLab antimony [https://colab.ws/articles/10.1007%2Fs10800-023-02022-7]
- 28. Alloy Embedded in Bismuth Matrix for Ultra-Stable... Antimony Carbon [https://pmc.ncbi.nlm.nih.gov/articles/PMC10051522/]
- 29. Paper-Based Working Electrodes Coated with Mercury or Bismuth Films for Heavy Metals Determination - PubMed [https://pubmed.ncbi.nlm.nih.gov/32414133/]
- 30. (PDF) Applicability of the Bismuth Bulk Rotating Disk Electrode for... [https://www.academia.edu/11687847/Applicability_of_the_Bismuth_Bulk_Rotating_Disk_Electrode_for_heavy_metal_monitoring_in_undisturbed_environmental_and_biological_samples_Determination_of_Zn_II_in_rainwater_tap_water_and_urine]
- 31. -Modified Hydroxyapatite Carbon Bismuth for Electrode ... Heavy Metal [https://www.scientific.net/AMM.625.813]
- 32. (PDF) Graphite-epoxy composite as an alternative material to design... [https://www.academia.edu/32239910/Graphite_epoxy_composite_as_an_alternative_material_to_design_mercury_free_working_electrodes_for_stripping_voltammetry]
- 33. metal analysis with Trace -state solid – Part 2 | Metrohm electrodes [https://www.metrohm.com/en/discover/blog/20-21/trace-metal-analysis-with-solid-state-electrodes---part-2.html]
- 34. Using Trace -State Solid for Metal Analysis (Part 2) Electrodes [https://www.azom.com/article.aspx?ArticleID=19840]
- 35. scTRACE Gold [https://www.labbulletin.com/articles/20140108]
- 36. Anodic stripping voltammetry with gold electrodes as an alternative method for the routine determination of mercury in fish. Comparison with spectroscopic approaches - PubMed [https://pubmed.ncbi.nlm.nih.gov/27979266/]
- 37. A Portable Setup for the Voltammetric Determination of Total Mercury in Fish with Solid and Nanostructured Gold Electrodes - PubMed [https://pubmed.ncbi.nlm.nih.gov/31109011/]
- 38. Sensors | Free Full-Text | Mercury Determination in Fish Samples by Chronopotentiometric Stripping Analysis Using Gold Electrodes Prepared from Recordable CDs | HTML [https://www.mdpi.com/1424-8220/8/11/7157/htm]
- 39. Trace metal analysis with solid-state electrodes – Part 3 | Metrohm [https://www.metrohm.com/en_us/discover/blog/20-21/trace-metal-analysis-with-solid-state-electrodes---part-3.html]
- 40. metal analysis with Trace -state solid – Part 3 electrodes [https://www.linkedin.com/pulse/trace-metal-analysis-solid-state-electrodes-part-3-lanciki-ph-d-]
- 41. RoHS Compliant | INCERTEC [https://incertec.com/rohs-compliant/]
- 42. Product Restrictions - International Antimony Association [https://www.antimony.com/regulations-compliance/product-restrictions/]
- 43. Mercury-free determination of heavy metals by ... [https://metrohm.com/en/discover/news/2023/mercury-free-determination-of-heavy-metals-by-voltammetric-trace.html]
- 44. of Analysis and Lead by Zinc - Mercury ... Free Potentiometric [https://orbit.dtu.dk/en/publications/analysis-of-lead-and-zinc-by-mercury-free-potentiometric-strippin/]
- 45. Investigation of mercury - free and... potentiometric stripping analysis [https://link.springer.com/article/10.1007/s002160050626]
- 46. Mercury-free disposable lead sensors based on potentiometric stripping analysis at gold-coated screen-printed electrodes - PubMed [https://pubmed.ncbi.nlm.nih.gov/8328670/]
- 47. Zinc detection in oil-polluted marine environment by stripping voltammetry with mercury-free nanoporous gold electrode | Scientific Reports [https://www.nature.com/articles/s41598-022-20067-0]
- 48. Gold-based screen-printed sensor for detection of trace lead - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0925400505005502]
- 49. Comparison Between Potentiometric and Stripping Voltammetric Detection of Trace Metals: Measurements of Cadmium and Lead in the Presence of Thalium, Indium, and Tin - PMC [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2836769/]
- 50. Bismuth-modified electrodes for lead detection - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S016599361000244X]
- 51. Bismuth electrodes in contemporary electroanalysis - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S2451910317300352]
- 52. (PDF) Bismuth film electrode for simultaneous adsorptive stripping ... [https://www.academia.edu/30099330/Bismuth_film_electrode_for_simultaneous_adsorptive_stripping_analysis_of_trace_cobalt_and_nickel_using_constant_current_chronopotentiometric_and_voltammetric_protocol]
- 53. Inexpensive Bismuth -Film Electrode Supported on Pencil- Lead ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6196922/]
- 54. of heavy metals in tap water using the Stripping film... analysis bismuth [https://link.springer.com/article/10.1007/s00216-009-3294-7]
- 55. Using nanostructured conductive carbon tape modified with bismuth ... [https://pubmed.ncbi.nlm.nih.gov/24054585/]
- 56. (PDF) The Influence of the Electrodeposition Conditions on the... [https://www.academia.edu/13172110/The_Influence_of_the_Electrodeposition_Conditions_on_the_Electroanalytical_Performance_of_the_Bismuth_Film_Electrode_for_Lead_Determination]
- 57. Optimization and validation of the methods for the total mercury and... [https://pubmed.ncbi.nlm.nih.gov/28340739/]
- 58. Assessment of Levels of Mercury in Human Breast in... | IIETA Milk [https://iieta.org/journals/eesrj/paper/10.18280/eesrj.070301]
- 59. Methylmercury determination in freshwater biota and sediments: Static headspace GC-MS compared to direct mercury analyzer - PMC [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8720905/]
- 60. Methylmercury determination in freshwater biota and sediments: Static headspace GC-MS compared to direct mercury analyzer - ScienceDirect [https://www.sciencedirect.com/science/article/pii/S221501612100371X]
- 61. The mercury concentration in breast milk resulting from amalgam fillings and dietary habits - PubMed [https://pubmed.ncbi.nlm.nih.gov/9600805/]
- 62. of Comparison Methods of Compound Levels and Analysis ... Mercury [https://scispace.com/papers/comparison-of-analysis-methods-of-compound-levels-and-199idyb0]
- 63. voltammetry with a bismuth Stripping as an electrode to... alternative [https://www.sciepub.com/WJCE/abstract/15464]
- 64. Recent developments in on-line electrochemical stripping analysis—An overview of the last 12 years - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0003267010013036]
- 65. Adsorption of adenine on mercury in acetate buffer at electrode ... pH [https://pmc.ncbi.nlm.nih.gov/articles/PMC6060954/]
- 66. Electrochemical determination of closantel in the commercial... [https://link.springer.com/article/10.1007/s00706-016-1862-z]
- 67. BASi® | FAQ: EC Electrodes [https://www.basinc.com/products/ec/faqele]
- 68. Compensate for or Minimize Matrix Effects? Strategies for Overcoming Matrix Effects in Liquid Chromatography-Mass Spectrometry Technique: A Tutorial Review - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7412464/]
- 69. Strategies for the Detection and Elimination of Matrix Effects in Quantitative LC–MS Analysis | LCGC International [https://www.chromatographyonline.com/view/strategies-detection-and-elimination-matrix-effects-quantitative-lc-ms-analysis]
- 70. study should be performed for every Interference ... protein [https://link.springer.com/article/10.1007/s007690100383]
- 71. Adsorptive potentiometric stripping of nucleic acids at... analysis [https://repositorio.unesp.br/entities/publication/d757fd28-c5a1-4115-a344-720de103b329]
- 72. sciencedirect.com/topics/chemistry/anodic- stripping - voltammetry [https://www.sciencedirect.com/topics/chemistry/anodic-stripping-voltammetry]
- 73. of Stripping using gold analyses : irreversible... mercury electrodes [https://pubmed.ncbi.nlm.nih.gov/21662908/]
- 74. cathodic stripping voltammetry: Topics by Science.gov [https://www.science.gov/topicpages/c/cathodic+stripping+voltammetry]
- 75. Adsorptive Stripping Voltammetry - an overview | ScienceDirect Topics [https://www.sciencedirect.com/topics/chemistry/adsorptive-stripping-voltammetry]
- 76. Electrodes with extremely high hydrogen overvoltages as substrate electrodes for stripping analysis based on bismuth-coated electrodes - PubMed [https://pubmed.ncbi.nlm.nih.gov/22790698/]
- 77. Endovascular neural stimulation with platinum and platinum black modified electrodes - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11926064/]
- 78. Frontiers | Protocol for Screening Water Oxidation or Reduction Electrocatalyst Activity in a Three-Electrode Cell for Alkaline Exchange Membrane Electrolysis [https://www.frontiersin.org/journals/energy-research/articles/10.3389/fenrg.2022.871604/full]
- 79. Electrochemistry for API Synthesis [https://www.pharmtech.com/view/electrochemistry-for-api-synthesis]
- 80. Economic bismuth-film microsensor for anodic stripping of... analysis [https://colab.ws/articles/10.1007%2Fs00216-005-0083-9]
- 81. Trace vanadium analysis by catalytic adsorptive stripping voltammetry... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2707498/]
- 82. How to select the right reference electrode? - BioLogic Learning Center [https://www.biologic.net/topics/how-to-select-the-right-reference-electrode/]
- 83. (PDF) Mercury -Free PSA of Heavy Metals Using Graphite-Epoxy... [https://www.academia.edu/50370285/Mercury_Free_PSA_of_Heavy_Metals_Using_Graphite_Epoxy_Composite_Electrodes]
- 84. Comparison of linear scan and differential pulse anodic stripping voltammetry at a thin mercury film glassy carbon electrode - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0003267001936207]
- 85. Anodic stripping voltammetry with gold electrodes as an alternative method for the routine determination of mercury in fish. Comparison with spectroscopic approaches - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0308814616319604]
- 86. Chromatographic Measurements, Part 5: Determining LOD and LOQ ... [https://www.sepscience.com/hplc-solutions-126-chromatographic-measurements-part-5-determining-lod-and-loq-based-on-the-calibration-curve-6959]
- 87. How to determine the LOD using the calibration curve? [https://mpl.loesungsfabrik.de/en/english-blog/method-validation/calibration-line-procedure]
- 88. Establishing Acceptance Criteria for Analytical Methods | BioPharm International [https://www.biopharminternational.com/view/establishing-acceptance-criteria-analytical-methods]
- 89. Using Trace Solid-State Electrodes for Metal Analysis (Part 4) [https://www.azom.com/article.aspx?ArticleID=19842]
- 90. From mercury to nanosensors: Past, present and the future perspective of electrochemistry in pharmaceutical and biomedical analysis - PubMed [https://pubmed.ncbi.nlm.nih.gov/27210510/]
- 91. From mercury to nanosensors: Past, present and the future perspective of electrochemistry in pharmaceutical and biomedical analysis - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0731708516302321]
- 92. Zinc detection in oil-polluted marine environment by stripping ... [https://www.nature.com/articles/s41598-022-20067-0?error=cookies_not_supported]
- 93. Atomic Absorption Spectrophotometry ( AAS ): Principles... [https://www.sepscience.com/atomic-absorption-spectrophotometry-aas-principles-instrumentation-and-comparisons-with-other-as-techniques-12134]
- 94. ICP-MS vs. AAS - Which Technique to Choose - Drawell [https://www.drawellanalytical.com/icp-ms-vs-aas-which-technique-to-choose/]
- 95. Prime Scientific - Comparison Between ICP and Atomic Absorption as Analytical Techniques [https://prime.erpnext.com/blog/application-knowledge/comparison-between-icp-and-atomic-absorption-as-analytical-techniques]
- 96. of the Performance of Comparison - ICP , CV-ICP-OES, and TDA... MS [https://pmc.ncbi.nlm.nih.gov/articles/PMC11656208/]
- 97. What Does Good Laboratory Practice (GLP) Mean? | LCGC International [https://www.chromatographyonline.com/view/what-does-good-laboratory-practice-glp-mean]
- 98. A Guide to Good ( Laboratory ) | SafetyCulture Practice GLP [https://safetyculture.com/topics/good-laboratory-practice-glp]
- 99. GMP, GLP and GCP differences [https://www.linkedin.com/pulse/glp-gcp-gmp-differences-comprehensive-guide-kymos-pharma-services-bxi6f]
- 100. GMP vs. GLP in Laboratory Testing & Validation [https://www.thefdagroup.com/blog/gmp-vs.-glp-in-lab-testing-validation]
- 101. Tools and Techniques for GLP-1 Analysis | LCGC International [https://www.chromatographyonline.com/view/tools-and-techniques-for-glp-1-analysis]