Current vs. Potential: A Guide to Voltammetry and Potentiometry for Pharmaceutical Analysis
This article provides a comprehensive comparison of voltammetry and potentiometry, two cornerstone electrochemical techniques in pharmaceutical research and drug development.
Current vs. Potential: A Guide to Voltammetry and Potentiometry for Pharmaceutical Analysis
Abstract
This article provides a comprehensive comparison of voltammetry and potentiometry, two cornerstone electrochemical techniques in pharmaceutical research and drug development. It explores their foundational principles, focusing on the measurement of current under an applied potential versus the measurement of potential at zero current. The scope extends to methodological applications in drug quantification, metabolite monitoring, and ion analysis, alongside practical troubleshooting for sensor optimization. A direct comparative analysis equips scientists with the knowledge to select the appropriate technique, highlighting how their complementary strengths are advancing therapeutic drug monitoring, point-of-care diagnostics, and personalized medicine.
Core Principles: Understanding Voltammetry's Current and Potentiometry's Potential
Electrochemical analysis encompasses a powerful suite of techniques for quantifying analytes, studying reaction mechanisms, and monitoring processes in fields ranging from drug development to environmental science. Among these techniques, potentiometry and voltammetry represent two fundamental, yet philosophically distinct, approaches. The core distinction lies in what is controlled and what is measured. Potentiometry is a static, equilibrium technique that measures a potential difference (voltage) at zero current flow to determine analyte activity. In contrast, voltammetry is a dynamic, non-equilibrium technique that applies a controlled potential profile and measures the resulting current response due to faradaic reactions [1] [2]. This whitepaper provides an in-depth technical guide to these methods, framing them within the broader context of research into signal generation and measurement. We will dissect their theoretical foundations, experimental protocols, and applications, with a particular emphasis on the critical relationship between the controlled signal and the measured response for each technique.
Theoretical Foundations and Core Principles
Potentiometry: Measuring Potential at Equilibrium
Potentiometry is defined as the measurement of an electrical potential (electromotive force) between two electrodes in an electrochemical cell when the net current flowing through the cell is zero or negligible [3] [4]. This zero-current condition is crucial as it ensures the measurement does not alter the solution's composition through electrolysis, making it an equilibrium technique [2].
The fundamental setup involves two electrodes: a reference electrode, which maintains a stable, known potential, and an indicator electrode (or ion-selective electrode, ISE), which develops a potential that depends on the activity (concentration) of a specific ion in the solution [5] [3]. The potential difference between these two electrodes is related to the analyte's activity by the Nernst equation [1] [5] [3]. For a monovalent ion, the relationship is:
E = E° + (0.0592/n) * log(a)
Where E is the measured potential, E° is the standard electrode potential, n is the number of electrons transferred, and a is the ion activity. This equation predicts a linear relationship between the measured potential and the logarithm of the ion activity, with a slope of approximately 59.2 mV per decade for a monovalent ion at 25°C [3]. The potential generated in ISEs is a phase boundary potential resulting from the selective transfer of ions across a membrane, not directly from a redox reaction [3].
Voltammetry: Measuring Current from Applied Potential
Voltammetry encompasses a group of techniques where the current flowing through an electrochemical cell is measured as a function of the applied potential to the working electrode [1] [2]. Unlike potentiometry, voltammetry is a dynamic technique that intentionally drives faradaic reactions (electron transfer) at the working electrode surface.
The applied potential provides the driving force for the oxidation or reduction of an analyte. The resulting faradaic current is proportional to the rate of this electron transfer reaction and, under controlled conditions, to the concentration of the analyte in the bulk solution [1] [2]. The current is limited by mass transport—the process by which analyte molecules diffuse from the bulk solution to the electrode surface to replenish those that have reacted. A key challenge in voltammetry is distinguishing the faradaic current from the capacitive current (or charging current), which arises from the charging and discharging of the electrical double layer at the electrode-solution interface and does not involve a chemical reaction [2]. Advanced voltammetric techniques like Differential Pulse Voltammetry (DPV) and Square Wave Voltammetry (SWV) are specifically designed to minimize the contribution of this capacitive current, thereby enhancing the signal-to-noise ratio for trace-level analysis [1] [6].
Table 1: Core Principles of Potentiometry and Voltammetry
| Feature | Potentiometry | Voltammetry |
|---|---|---|
| Controlled Parameter | Current (held at zero) | Potential (systematically varied) |
| Measured Signal | Potential (Voltage, E) | Current (I) |
| Cell State | Equilibrium (Static) | Non-Equilibrium (Dynamic) |
| Fundamental Equation | Nernst Equation | Fick's Laws of Diffusion & Faraday's Law |
| Primary Signal Source | Selective ion partitioning across a membrane | Electron transfer (redox) reaction rate |
| Typical Electrode Setup | Two-electrode (Reference & Indicator) | Three-electrode (Working, Reference, & Counter) |
Experimental Setups and Methodologies
The Potentiometric Cell and Electrode Types
A basic potentiometric cell requires two electrodes immersed in the sample solution [3]. The reference electrode (e.g., Ag/AgCl) provides a constant, stable potential against which changes are measured [1]. The indicator electrode responds selectively to the ion of interest. Key types of indicator electrodes include:
- Glass Membrane Electrodes: Used primarily for pH measurement and, with specific glass compositions, for sodium ions [3].
- Polymer Membrane Electrodes (ISEs): These use a poly(vinyl chloride) membrane impregnated with an ionophore (a selective ion-binding molecule) and a plasticizer. They are widely used for ions like K⁺, Ca²⁺, Li⁺, and Cl⁻ in clinical and environmental analysis [7] [3].
- Solid-State Electrodes: Employ a crystalline membrane, such as LaF₃ for fluoride ion detection [8].
A critical modern trend is the move toward solid-contact ion-selective electrodes (SC-ISEs), which eliminate the internal filling solution of traditional ISEs. This is achieved using a solid-contact layer (e.g., conducting polymers or carbon-based nanomaterials like MXenes or carbon nanotubes) that acts as an ion-to-electron transducer. This design enables easier miniaturization, better portability, and enhanced stability, which is vital for point-of-care devices and wearable sensors [7].
The Voltammetric Cell and Key Techniques
The standard voltammetric setup is a three-electrode system [1] [2]. The working electrode (e.g., glassy carbon, gold, mercury) is where the redox reaction of interest occurs. Its potential is precisely controlled relative to the reference electrode. The counter (or auxiliary) electrode (e.g., platinum wire) completes the electrical circuit, carrying the current so that no net current flows through the reference electrode, thus preserving its stable potential [1]. This arrangement, managed by a potentiostat, allows for precise control of the working electrode potential.
Common voltammetric techniques include:
- Cyclic Voltammetry (CV): The potential is scanned linearly in a forward and reverse direction. It is a primary tool for studying electrode reaction mechanisms, kinetics, and reversibility [1].
- Differential Pulse Voltammetry (DPV): Small potential pulses are superimposed on a linear baseline. Current is sampled just before and at the end of each pulse, and the difference is plotted. This minimizes capacitive current, making DPV highly sensitive for trace quantitative analysis [1] [6].
- Square Wave Voltammetry (SWV): Another pulsed technique with a square-wave modulation, offering very fast scans and high sensitivity [1].
- Anodic Stripping Voltammetry (ASV): An extremely sensitive technique for metal ions. The analyte is first electroplated (pre-concentrated) onto the working electrode at a constant potential. Subsequently, it is stripped off (re-dissolved) by scanning the potential, producing a sharp, measurable current peak [9].
Diagram 1: DPV Experimental Workflow
The Scientist's Toolkit: Essential Materials and Reagents
Successful implementation of these electrochemical methods relies on a suite of specialized materials and reagents.
Table 2: Key Research Reagent Solutions and Materials
| Item | Function/Description | Common Examples |
|---|---|---|
| Potentiostat/Galvanostat | Instrument that controls potential (potentiostat) or current (galvanostat) and measures the resulting electrochemical signal [10]. | Commercial benchtop systems, portable/pocket potentiostats. |
| Reference Electrode | Provides a stable, known reference potential for both potentiometric and voltammetric measurements [1]. | Ag/AgCl (Silver/Silver Chloride), SCE (Saturated Calomel Electrode). |
| Ion-Selective Membrane | The heart of an ISE; selectively binds the target ion, generating a membrane potential [3]. | PVC membranes with ionophores (e.g., valinomycin for K⁺), LaF₃ crystals (for F⁻). |
| Working Electrodes | The electrode where the reaction of interest occurs; material choice depends on the application and potential window [1]. | Glassy Carbon (GC), Gold, Platinum, Mercury (e.g., HMDE). |
| Solid-Contact Materials | Transduce ionic signal to electronic current in solid-contact ISEs; crucial for miniaturization [7]. | Conducting Polymers (e.g., PEDOT), Carbon Nanotubes (CNTs), Graphene. |
| Supporting Electrolyte | Carries current in solution and minimizes migration of the analyte; ensures the reaction is diffusion-controlled [2]. | Inert salts (e.g., KCl, KNO₃, phosphate buffers). |
| Nanomaterial Modifiers | Enhance electrode sensitivity, selectivity, and stability by increasing surface area and providing binding sites [9]. | Carbon nanotubes (SWCNTs/MWCNTs), metal nanoparticles (Au, Pt), Metal-Organic Frameworks (MOFs). |
Advanced Applications and Current Research Trends
Potentiometry in Clinical and Biomedical Sensing
Potentiometry has evolved far beyond the traditional pH meter. A significant trend is its integration into wearable sensors for the continuous monitoring of biomarkers, electrolytes, and even pharmaceuticals in biological fluids like sweat and interstitial fluid [7]. For instance, solid-contact ISEs are being developed for monitoring sodium and potassium levels in athletes or patients. Therapeutic Drug Monitoring (TDM) is another critical application, where potentiometric sensors measure drug concentrations in biofluids, which is especially vital for pharmaceuticals with a narrow therapeutic index [7]. Furthermore, the use of 3D printing and the development of low-cost, disposable paper-based potentiometric sensors are opening new avenues for rapid, in-field (point-of-care) diagnostic testing [7].
Voltammetry in Trace Analysis and Environmental Monitoring
Voltammetry excels in the sensitive and selective detection of electroactive species. Its pulse techniques (DPV, SWV) and stripping methods (ASV) are workhorses for trace metal analysis in environmental samples [1] [9]. Recent research focuses on enhancing these methods with nanomaterials to create advanced sensors. For example, electrodes modified with carbon nanotubes, graphene, or metal-organic frameworks (MOFs) exhibit improved sensitivity and selectivity for heavy metals like Pb²⁺, Cd²⁺, and Hg²⁺ in water and soil [9]. These nanomaterial-based voltammetric sensors offer a portable, cost-effective alternative to traditional lab-based methods like ICP-MS for real-time, on-site environmental monitoring [9]. In pharmaceutical research, voltammetry is routinely used for drug quantification and studying drug metabolism pathways.
Table 3: Comparison of Analytical Performance and Applications
| Aspect | Potentiometry | Voltammetry (e.g., DPV/ASV) |
|---|---|---|
| Typical Detection Limit | ~10⁻⁶ to 10⁻⁸ M [7] | ~10⁻⁸ to 10⁻¹¹ M (especially with stripping) [9] |
| Selectivity | High (from ionophore in membrane) | Moderate to High (from potential & electrode modification) |
| Primary Applications | Clinical electrolytes (Na⁺, K⁺, Cl⁻), pH, environmental monitoring (NO₃⁻, NH₄⁺) [5] [7] [3] | Trace metal analysis, drug compound quantification, redox mechanism studies [1] [9] |
| Sample Throughput | High (suitable for continuous monitoring) | Moderate (scanning takes time) |
| Miniaturization & Portability | Excellent (solid-contact ISEs, wearables) [7] | Good (hand-held potentiostats available) |
Potentiometry and voltammetry are two pillars of modern electroanalytical chemistry, each defined by a distinct paradigm of signal measurement. Potentiometry, as a zero-current technique, provides a direct measure of ion activity through potential readings, governed by the Nernst equation. Its strength lies in its simplicity, selectivity, and suitability for continuous monitoring, as evidenced by its dominant role in clinical electrolyte analysis and its emerging applications in wearable sensors. Voltammetry, a controlled-potential technique, derives its analytical power from measuring the faradaic current resulting from forced redox reactions. Its dynamic nature makes it exceptionally powerful for trace-level quantitative analysis, mechanistic studies, and environmental sensing, particularly when coupled with advanced pulse techniques and nanomaterial-enhanced electrodes. The choice between these techniques is not a matter of superiority but of strategic alignment with the analytical problem at hand—whether the research question is best answered by an equilibrium measurement of ion activity or by a dynamic probe of electron transfer kinetics and concentration. For drug development professionals and researchers, a deep understanding of these defining signals is essential for selecting the optimal tool to unlock critical chemical information.
Voltammetry represents a cornerstone of electroanalytical chemistry, dedicated to studying the current response generated by an electrochemical cell as a function of an applied potential. This technique provides a powerful platform for quantifying electroactive species, offering high sensitivity, rapid response, and detailed insights into electron transfer kinetics and reaction mechanisms. The fundamental principle involves applying a controlled, time-varying potential to a working electrode within an electrochemical cell and measuring the resulting current, which is proportional to the concentration of the analyte undergoing oxidation or reduction [11] [12]. The resulting plot of current versus applied potential, known as a voltammogram, serves as a unique fingerprint for analyte identification and quantification [12].
This guide is framed within a broader research context contrasting voltammetry with potentiometry, another primary electroanalytical technique. While voltammetry measures current at a controlled potential, potentiometry measures the potential (voltage) of an electrochemical cell at near-zero current to determine ion activity [7] [5]. Potentiometry is renowned for its simplicity and direct readout of ion concentrations via the Nernst equation, making it ideal for continuous monitoring and portable ion-selective electrodes [7]. In contrast, voltammetry excels in sensitivity for trace-level analysis, the ability to detect multiple analytes simultaneously, and the provision of rich information about reaction kinetics and thermodynamics, making it indispensable for complex analytical challenges in drug development, neuroscience, and environmental monitoring [9] [12].
Fundamental Principles of the Voltammetric Cell
Core Components and the Signal Generation Process
At the heart of voltammetry is the voltammetric cell, a system designed to facilitate and measure electrochemical reactions. The following diagram illustrates the core signaling pathway and the relationship between the applied potential and the measured faradaic current.
The process begins when a potential waveform is applied to the working electrode. This potential provides the energy necessary to drive the transfer of electrons between the electrode surface and the target analyte in solution [12]. This electron transfer induces a redox reaction in the analyte, either oxidizing it (loss of electrons) or reducing it (gain of electrons). The rate of this electron transfer is directly controlled by the applied potential. The movement of electrons to or from the electrode constitutes a faradaic current, which is the primary analytical signal in voltammetry [11]. This current is measured and plotted against the applied potential to produce a voltammogram, which the instrument's system can use for further control and analysis.
Essential Components of a Voltammetric Cell
A typical voltammetric cell operates with a three-electrode configuration, which offers superior potential control compared to a two-electrode system. The key components are detailed below.
- Working Electrode (WE): This is the electrode where the reaction of interest occurs. Its material is chosen based on the analyte and the required potential window. Common materials include glassy carbon, gold, platinum, and carbon-based materials like carbon nanotubes or graphene [9] [11]. The surface of the working electrode is often modified with nanomaterials or polymers to enhance sensitivity and selectivity [11].
- Counter Electrode (CE) (or Auxiliary Electrode): This electrode, often made of an inert wire like platinum, completes the electrical circuit. It allows current to flow through the cell, ensuring that the current measured at the working electrode is solely due to the redox reactions occurring at its surface.
- Reference Electrode (RE): This electrode (e.g., Ag/AgCl) maintains a stable, known, and constant potential throughout the experiment [7]. It serves as a reference point against which the potential of the working electrode is precisely controlled and measured, ensuring the accuracy and reproducibility of the applied potential waveform.
- Electrolyte Solution: The cell contains a solution of an electrolyte (e.g., KCl, phosphate buffer) in which the analyte is dissolved. This electrolyte is chemically inert over the potential range of interest and has a much higher concentration than the analyte. Its primary function is to carry the current between the electrodes by ionic conduction, minimizing the solution's electrical resistance.
Key Voltammetric Techniques and Methodologies
Voltammetry encompasses a family of techniques, each defined by its specific applied potential waveform, which is tailored to optimize sensitivity, selectivity, or speed for different analytical scenarios.
Common Voltammetric Techniques
Table 1: Overview of Common Voltammetric Techniques
| Technique | Waveform Description | Key Features and Output | Primary Applications |
|---|---|---|---|
| Cyclic Voltammetry (CV) [11] [13] [12] | Potential is swept linearly in a forward and reverse direction. | Provides information on reaction reversibility, redox potentials, and electron transfer kinetics. Produces peaks for oxidation and reduction. | Studying reaction mechanisms, characterizing modified electrodes, determining formal potentials. |
| Differential Pulse Voltammetry (DPV) [9] [11] [12] | Small amplitude pulses superimposed on a linear base potential. | High sensitivity and low detection limits by minimizing charging (capacitive) current. Produces a peak-shaped voltammogram. | Trace-level detection of analytes in complex matrices (e.g., biomarkers, heavy metals). |
| Square Wave Voltammetry (SWV) [14] [11] [12] | A square wave is superimposed on a staircase waveform. | Very fast and highly sensitive. Effectively rejects capacitive current by measuring the difference between forward and reverse currents. | Rapid, sensitive detection for biosensing and trace analysis. |
| Anodic Stripping Voltammetry (ASV) [9] [12] | Two-step: Preconcentration at a negative potential, followed by an anodic (positive) potential sweep. | Extremely low detection limits (parts-per-trillion) for metals. | Ultra-trace heavy metal analysis (e.g., Pb, Cd, Hg) in environmental and biological samples. |
| Normal Pulse Voltammetry (NPV) [15] [12] | Series of increasing potential pulses of short duration applied to a constant initial potential. | Minimizes capacitive current by measuring current at the end of each pulse. | Analytical measurements where minimizing capacitive contributions is critical. |
Experimental Protocol: Representative Voltammetric Workflow
The following diagram and protocol outline a general workflow for a voltammetric experiment, which can be adapted for specific techniques like CV or DPV.
- Cell Assembly and Electrode Preparation: Clean the working electrode according to standard protocols (e.g., polishing on a microcloth with alumina slurry for glassy carbon). Rinse thoroughly with deionized water. Assemble the three-electrode system in the electrochemical cell, ensuring proper placement of the working, counter, and reference electrodes [11].
- Introduction of Electrolyte and Deaeration: Fill the cell with the appropriate electrolyte solution. For experiments involving reducible analytes (e.g., oxygen), purge the solution with an inert gas like nitrogen or argon for at least 15-20 minutes to remove dissolved oxygen, which can interfere by undergoing reduction. Maintain a blanket of gas over the solution during measurement.
- System Calibration and Validation (Optional but recommended): Perform a calibration using a standard solution of a known redox couple, such as potassium ferricyanide, to verify the system's performance and the cleanliness of the electrode. In Cyclic Voltammetry, this confirms the redox potential and reversibility of the standard.
- Introduction of Analyte: Introduce the analyte of interest into the cell, typically via micropipette. Stir the solution gently (if using magnetic stirring) to ensure homogeneous distribution. Allow the solution to become quiescent before measurement if stirring is not part of the technique.
- Application of Potential Waveform and Data Acquisition: Using the potentiostat, select and configure the desired voltammetric technique (e.g., CV, DPV, SWV) and its parameters (e.g., initial/final potential, scan rate, pulse amplitude, frequency). Initiate the experiment. The potentiostat will apply the waveform and record the current response, generating the voltammogram in real-time.
- Data Analysis and Interpretation: Analyze the resulting voltammogram. Identify peak currents and potentials. For quantitative analysis, construct a calibration curve by measuring the peak current at different analyte concentrations. For kinetic studies, analyze how peak currents and potentials shift with scan rate.
The Scientist's Toolkit: Essential Research Reagents and Materials
The performance of a voltammetric sensor is highly dependent on the materials used in its construction, particularly the working electrode and its modifications.
Table 2: Key Materials for Voltammetric Sensor Development
| Category | Specific Examples | Function in the Voltammetric Cell |
|---|---|---|
| Electrode Materials | Glassy Carbon (GC), Gold (Au), Platinum (Pt) [11] | Provide a conductive, electroactive surface for electron transfer. Choice depends on potential window and analyte reactivity. |
| Carbon Nanomaterials | Carbon Nanotubes (CNTs), Graphene, Graphene Oxide (GO) [9] [11] | Enhance electron transfer kinetics, increase electrode surface area, and improve sensitivity due to their high conductivity and unique structures. |
| Metal & Metal Oxide Nanoparticles | Gold Nanoparticles (AuNPs), Silver Nanoparticles (AgNPs), Fe3O4, ZnO [9] [11] | Provide electrocatalytic activity, reduce overpotentials for redox reactions, and can be used for biomolecule immobilization. |
| Polymers & Composites | Poly(3,4-ethylenedioxythiophene) (PEDOT), Polyaniline, Chitosan-based composites [7] [11] | Used as permselective membranes to block interferents, for immobilization of recognition elements (enzymes, aptamers), or as ion-to-electron transducers. |
| Supporting Electrolytes | Potassium Chloride (KCl), Phosphate Buffered Saline (PBS), Sodium Perchlorate (NaClO4) [13] | Carry ionic current, control ionic strength, and fix the pH of the solution to ensure the electrochemical reaction is not limited by solution resistance. |
| Redox Mediators / Standards | Potassium Ferricyanide ([Fe(CN)₆]³⁻/⁴⁻), Ferrocenedimethanol (Fc(MeOH)₂ [13] | Used for system validation, electrode characterization, and to study electron transfer kinetics. Also used as labels or mediators in biosensors. |
Advanced Applications and Future Perspectives
Voltammetric cells have moved beyond basic research and are now pivotal in cutting-edge applications. In neurochemical monitoring, carbon-fiber microelectrodes used with fast-scan cyclic voltammetry (FSCV) enable real-time, in vivo detection of neurotransmitters like dopamine with high spatiotemporal resolution [12]. In pharmaceutical analysis, voltammetry is used for therapeutic drug monitoring (TDM) of pharmaceuticals with narrow therapeutic indices, offering a rapid alternative to traditional chromatographic methods [7] [12]. In environmental monitoring, techniques like ASV are indispensable for detecting trace heavy metals such as lead, cadmium, and mercury in water samples at parts-per-trillion levels [9] [12].
The future of voltammetry is being shaped by several key trends. The integration of nanomaterials like MXenes, metal-organic frameworks (MOFs), and hybrid nanocomposites continues to push the boundaries of sensor sensitivity and selectivity [9] [11]. There is a growing emphasis on miniaturization and portability, with the development of wearable sensors and 3D-printed electrode platforms for point-of-care testing and field analysis [7]. Finally, the fusion of voltammetry with digital technologies, such as artificial intelligence and machine learning, is beginning to automate signal processing, enable adaptive recalibration, and extract complex patterns from multivariate voltammetric data, opening new frontiers in intelligent sensing [11].
Potentiometry is a cornerstone electrochemical technique characterized by its operation at zero-current conditions. Unlike dynamic methods like voltammetry that measure current from electron transfer, potentiometry passively measures the potential difference, or electromotive force (EMF), between two electrodes to determine the activity of target ions in solution [7] [16]. This fundamental distinction makes it a powerful tool for direct, non-destructive chemical analysis.
The technique's significance is underscored by its diverse applicability across clinical diagnostics, environmental monitoring, pharmaceutical analysis, and industrial process control [7] [1]. Its principle is governed by the Nernst equation, which describes the relationship between the measured potential and the ionic activity of the analyte [17]. A core advantage of this zero-current approach is its minimal consumption of the analyte and relative insensitivity to solution turbidity or color, making it suitable for complex real-world samples [7].
This guide details the core components, functioning, and experimental implementation of potentiometric cells, framing this discussion within a broader research context that contrasts potential measurement in potentiometry with current measurement in voltammetry.
Core Components of a Potentiometric Cell
A typical potentiometric cell consists of two primary electrodes immersed in the sample solution, completing an electrochemical cell where the potential is measured without significant current flow [17].
The Indicator Electrode
The indicator (or working) electrode's potential varies in response to the activity of the specific ion of interest. The most common types are Ion-Selective Electrodes (ISEs), which incorporate a membrane designed to be selective for a particular ion [7] [1].
- Membrane Types: ISEs use various membrane materials to achieve selectivity, including glass (for H⁺, Na⁺), crystalline solids, or polymeric membranes impregnated with ionophores (ion-binding molecules) [18].
- Response Mechanism: The potential develops across the ion-selective membrane due to the unequal distribution of ions between the sample and the membrane phase, a process governed by the Nernst equation [17].
The Reference Electrode
The reference electrode provides a stable, known, and constant potential against which the indicator electrode's potential is measured [1] [17]. Its stability is critical for the accuracy of the entire measurement.
- Common Types: The Ag/AgCl (silver/silver chloride) and saturated calomel (SCE) electrodes are most frequently used [1] [19].
- Key Feature: A reference electrode maintains a constant potential by employing a reversible half-cell containing an electrolyte of fixed composition [17]. A salt bridge (often filled with concentrated KCl) facilitates ionic contact with the sample solution while minimizing the mixing of electrolytes [17].
Table 1: Key Electrodes in a Potentiometric Cell
| Electrode Type | Function | Key Characteristics | Common Examples |
|---|---|---|---|
| Indicator Electrode | Responds to the activity of a specific ion in the sample solution. | Potential changes log-linearly with ion activity (Nernstian response). High selectivity for target ion. | pH glass electrode; Ca²⁺, K⁺, Pb²⁺ ion-selective electrodes [1] [20]. |
| Reference Electrode | Provides a stable, known reference potential for measurement. | Constant electrochemical potential; unaffected by sample composition. | Ag/AgCl electrode; Saturated Calomel Electrode (SCE) [1] [19]. |
Cell Diagram and Potential Development
The overall cell potential (E~cell~) is the difference between the potentials of the indicator and reference electrodes: E~cell~ = E~ind~ − E~ref~ [19]. This measured potential, E~cell~, is related to the analyte's activity (a~I~) by the Nernst equation: E = E⁰ + (2.303RT/zF) log(a~I~) where E⁰ is the standard electrode potential, R is the gas constant, T is temperature, z is the ion's charge, and F is the Faraday constant [17] [20].
Potentiometry vs. Voltammetry: A Core Methodological Contrast
The fundamental difference between potentiometry and voltammetry lies in what is controlled and what is measured, leading to distinct applications and information outputs [1] [10].
Potentiometry: This is a zero-current technique. The potential difference between the indicator and reference electrode is measured under conditions of thermodynamic equilibrium, with no significant current flowing through the cell [7] [16]. The output is a potential related to ionic activity by the Nernst equation. It is primarily used for direct determination of ion concentrations/activities [1].
Voltammetry: This is a controlled-potential technique. The current flowing through the cell is measured as the applied potential at the working electrode is systematically varied [1] [16]. This is a dynamic, non-equilibrium method where electron transfer (current flow) is central. It provides both quantitative and qualitative information about electroactive species, including their concentration, redox potentials, and reaction kinetics [1].
Table 2: Contrasting Potentiometry and Voltammetry
| Parameter | Potentiometry | Voltammetry |
|---|---|---|
| Controlled Variable | None (zero current) / Open Circuit Potential | Electrode Potential |
| Measured Signal | Potential (Volts) | Current (Amperes) |
| Current Flow | Negligible (Theoretical Zero) | Significant (Measured Directly) |
| Governing Equation | Nernst Equation | Nernst Equation & Fick's Laws of Diffusion |
| Primary Information | Ionic Activity / Concentration | Redox Behavior, Concentration, Kinetics |
| System State | Equilibrium / Near-Equilibrium | Non-Equilibrium / Dynamic |
| Common Electrode Setup | Two-Electrode (Indicator & Reference) | Three-Electrode (Working, Reference, & Counter) |
| Example Application | pH measurement, clinical electrolyte analysis [7] [1] | Trace metal detection, studying reaction mechanisms [1] |
Experimental Protocol: Potentiometric Titration for Iron Determination
Potentiometric titration showcases the application of a potentiometric cell to monitor the progress of a redox reaction, determining the endpoint without a visual indicator [19]. The following protocol details the determination of Fe²⁺ concentration using a potassium permanganate (KMnO₄) titrant.
Principle
A sample containing Fe²⁺ is titrated with a standardized KMnO₄ solution. The redox reaction between MnO₄⁻ and Fe²⁺ causes a change in the solution's potential. An indicator electrode (e.g., platinum) senses this change relative to a reference electrode. The endpoint is identified as the point of maximum potential change on a plot of measured potential (E~cell~) versus titrant volume [19].
Key Reaction: [ \ce{MnO4^{-} + 8H+ + 5Fe^{2+} -> Mn^{2+} + 5Fe^{3+} + 4H2O} ] The Nernst equation for the permanganate half-reaction is: [ E{PM} = E^{∘}{PM} - \frac{RT}{5F} \ln{\frac{[Mn^{2+}]}{[MnO_4^{-}][H^{+}]^8}} ] The cell potential is measured as E~cell~ = E~ind~ − E~ref~, where E~ind~ is the potential of the platinum indicator electrode and E~ref~ is the potential of a reference electrode like Ag/AgCl [19].
Materials and Reagents
Table 3: Research Reagent Solutions for Potentiometric Titration
| Reagent/Solution | Function / Role in the Experiment |
|---|---|
| Fe²⁺ Solution (Analyte) | The solution of unknown concentration to be determined. Acts as the reducing agent in the redox titration [19]. |
| KMnO₄ Solution (Titrant) | Standardized oxidizing agent of known concentration. Reacts stoichiometrically with the Fe²⁺ analyte [19]. |
| Platinum (Pt) Electrode | Serves as the indicator electrode. Its potential changes as the ratio of [Fe³⁺]/[Fe²⁺] (and [MnO₄⁻]/[Mn²⁺]) shifts during the titration [19]. |
| Ag/AgCl Reference Electrode | Provides a stable, known reference potential against which the Pt indicator electrode's potential is measured [19]. |
| Acid (e.g., H₂SO₄) | Provides the H⁺ ions required for the permanganate half-reaction, ensuring the reaction proceeds correctly and at a practical rate [19]. |
Step-by-Step Procedure
- Cell Assembly: Place a known volume of the Fe²⁺ sample solution into a clean beaker. Acidify it with sulfuric acid to provide the necessary H⁺ ions. Immerse the cleaned platinum indicator electrode and the Ag/AgCl reference electrode into the solution [19].
- Instrument Setup: Connect the electrodes to a high-impedance voltmeter (potentiometer). Ensure the solution is being stirred constantly using a magnetic stirrer to maintain homogeneity.
- Initial Measurement: Record the initial cell potential (EMF) and the initial burette reading of the KMnO₄ titrant.
- Titration and Data Collection: Begin adding the KMnO₄ titrant in small increments (e.g., 0.5-1.0 mL). After each addition, allow the potential to stabilize and then record the volume added and the corresponding cell potential. As the endpoint is approached (indicated by larger potential jumps), reduce the titrant increments to 0.1 or 0.2 mL.
- Post-Endpoint Data: Continue adding titrant and recording potential and volume for several mL after the endpoint to fully define the titration curve.
- Endpoint Determination: Plot the measured cell potential (E~cell~) against the volume of KMnO₄ titrant added. The equivalence point is identified as the volume at the steepest inflection point of the sigmoidal-shaped curve. This can be found precisely by calculating the first derivative (ΔE/ΔV) of the titration data.
- Calculation: Use the volume at the equivalence point (V~eq~), the known concentration of the KMnO₄ titrant (C~KMnO4~), and the stoichiometry of the reaction to calculate the moles and concentration of Fe²⁺ in the sample.
Advanced Sensor Architectures and Applications
The field of potentiometry has evolved significantly beyond traditional glass electrodes, with innovations enhancing performance in complex matrices.
Solid-Contact Ion-Selective Electrodes (SC-ISEs)
Modern research focuses on Solid-Contact ISEs (SC-ISEs), which eliminate the internal filling solution of traditional ISEs. This design offers superior mechanical stability, ease of miniaturization, and portability [7]. The solid-contact layer, situated between the ion-selective membrane and the electronic conductor, functions as an ion-to-electron transducer [7]. Common transducer materials include:
- Conducting Polymers: e.g., polyaniline, PEDOT [7].
- Nanomaterials: Carbon nanotubes, graphene, and metal-organic frameworks (MOFs) that provide high capacitance and stability [7].
- Nanocomposites: Materials like MoS₂ nanoflowers filled with Fe₃O₄ are engineered to create synergistic effects, preventing structural collapse and enhancing electrochemical characteristics [7].
Applications in Research and Industry
The versatility of potentiometric sensors is demonstrated by their wide-ranging applications:
- Clinical Diagnostics and Biomedical Applications: Continuous monitoring of electrolytes (Na⁺, K⁺, Ca²⁺, Cl⁻) and blood gases is vital in critical care [7] [1]. Wearable potentiometric sensors are emerging for non-invasive monitoring of biomarkers and electrolytes in sweat or interstitial fluid [7]. Therapeutic Drug Monitoring (TDM) is another crucial application, especially for pharmaceuticals with a narrow therapeutic index, where potentiometric sensors can track drug concentrations in biological fluids [7].
- Environmental and Industrial Monitoring: Potentiometric sensors are deployed for detecting heavy metals like lead (Pb²⁺), copper (Cu²⁺), and mercury (Hg²⁺) in water and soil [7] [20]. They are also used for determining anions such as nitrate (NO₃⁻) and chloride (Cl⁻), which are critical for assessing water quality and agricultural runoff [7]. In industry, they play a role in quality control for pharmaceuticals and detergent manufacturing [7] [21].
- Agro-Food and Forensic Sciences: Analysis of ions in soils, plant materials, and food products is a common application [21]. Potentiometry is also used in forensic analysis for detecting drugs or toxic substances at crime scenes with minimal sample preparation [7].
The potentiometric cell, operating on the fundamental principle of zero-current potential measurement, remains an indispensable tool in the analytical scientist's arsenal. Its simplicity, selectivity, and direct readout of ionic activity, as described by the Nernst equation, provide distinct advantages for quantitative analysis across diverse fields. The ongoing innovation in sensor design—particularly through solid-contact architectures, novel nanomaterials, and the development of wearable platforms—ensures that potentiometry will continue to be a vital technique for precise chemical sensing in both laboratory and real-world settings. By understanding its core principles and contrasting them with dynamic techniques like voltammetry, researchers can better select and optimize the appropriate electrochemical method for their specific analytical challenges.
In the realm of modern electroanalytical chemistry, particularly within pharmaceutical and bioanalytical research, two fundamental measurement paradigms exist: the measurement of current in voltammetry and the measurement of potential in potentiometry. These approaches form the cornerstone of quantitative analysis for diverse applications ranging from drug detection in biological matrices to environmental monitoring of pharmaceutical residues [22]. The distinction between these techniques is not merely operational but stems from fundamental differences in what is controlled and what is measured in the electrochemical cell [10].
Voltammetric techniques involve applying a time-dependent potential to an electrochemical cell and measuring the resulting current as a function of that potential [23]. This current signal is intrinsically linked to the rate of electron transfer and mass transport of analyte to the electrode surface. In contrast, potentiometry passively measures the potential of a solution between two electrodes at zero current, a measurement that relates to the thermodynamic activity of ions in solution [16]. This in-depth technical guide explores the core governing equations of these methods—the Randles-Ševčík equation for voltammetry and the Nernst equation for potentiometry—framed within the context of current versus potential measurement research for drug development applications.
The Nernst Equation: The Thermodynamic Foundation of Potentiometry
Fundamental Principles and Mathematical Formalism
Potentiometry is a zero-current technique that measures the potential difference between two electrodes (a reference electrode and an indicator electrode) when no net current is flowing through the cell [1] [16]. This measured potential is a direct function of the concentration or activity of a specific ion in the solution, as described by the Nernst equation. For a general redox reaction: $$Ox + ne^- \rightleftharpoons Red$$ the Nernst equation is expressed as: $$E = E^0 - \frac{RT}{nF} \ln\frac{a{Red}}{a{Ox}}$$ where (E) is the measured potential, (E^0) is the standard electrode potential, (R) is the universal gas constant, (T) is the absolute temperature, (n) is the number of electrons transferred in the half-reaction, (F) is the Faraday constant, and (a{Red}) and (a{Ox}) are the activities of the reduced and oxidized species, respectively [1].
In practice, for ion-selective electrodes (ISEs), the equation is often simplified to: $$E = E^0 - \frac{0.05916}{n} \log[a]$$ at 25°C, where ([a]) is the activity of the ion of interest [1]. A key advantage of potentiometric sensing is its non-destructive nature, as it virtually does not consume the analyte during measurement, making it particularly valuable for small sample volumes with low analyte concentrations [24].
Experimental Protocols and Applications in Drug Development
Ion-Selective Electrode (ISE) Methodology for Drug Ion Analysis The fundamental setup for potentiometric analysis involves an electrochemical cell with two electrodes: a reference electrode that provides a stable, known potential, and an indicator electrode whose potential changes with the sample's composition [16]. Ion-selective electrodes are designed to respond selectively to a single type of ion through incorporation of specific ionophores in the membrane [1].
- Electrode Preparation: For a custom ISE, a polyvinyl chloride (PVC) membrane is typically prepared containing a plasticizer (e.g., 2-nitrophenyloctyl ether), an ion exchanger (e.g., potassium tetrakis(p-Cl-phenyl)borate), and for enhanced selectivity, a neutral ionophore (e.g., dicyclohexyl-18-crown-6 for dopamine sensing) [24]. This membrane cocktail is dissolved in tetrahydrofuran (THF) and cast into a mold or directly applied to an electrode body.
- Measurement Protocol: The ISE and reference electrode are immersed in the sample solution. The potential difference between them is measured after stabilization, ensuring zero current conditions. The potential is recorded and related to the analyte concentration via a calibration curve constructed from standard solutions [1] [24].
- Data Analysis: A plot of (E) vs. (\log[a]) yields a straight line with a slope of approximately (59.16/n) mV/decade at 25°C. Deviations from this slope may indicate non-ideal behavior or issues with the electrode.
Potentiometry is invaluable in pharmaceutical research for electrolyte analysis in clinical labs, monitoring ionic drugs, and potentiometric titrations where the endpoint is determined by a sharp change in potential, providing greater accuracy than visual indicators [1]. Recent research explores novel ionophores for neurotransmitters like dopamine, aiming to overcome selectivity challenges against common interferences such as ascorbic and uric acids [24].
The Randles-Ševčík Equation: The Quantitative Bridge in Voltammetry
Fundamental Principles and Mathematical Formalism
Voltammetry is a dynamic technique that measures the current passing through an electrochemical cell as a function of the applied potential [1]. The resulting current-potential plot is called a voltammogram. For a reversible system at a planar macroelectrode, the peak current in linear sweep voltammetry (LSV) or cyclic voltammetry (CV) is described by the Randles-Ševčík equation: $$ip = (2.69 \times 10^5) \cdot n^{3/2} \cdot A \cdot D^{1/2} \cdot C \cdot \nu^{1/2}$$ where (ip) is the peak current (A), (n) is the number of electrons transferred, (A) is the electrode area (cm²), (D) is the diffusion coefficient (cm²/s), (C) is the bulk concentration (mol/cm³), and (\nu) is the scan rate (V/s) [23].
This equation highlights that the measured current is directly proportional to the analyte concentration and the square root of the scan rate, indicating a diffusion-controlled process. Unlike the thermodynamic relationship in potentiometry, the Randles-Ševčík equation deals with kinetics and mass transport. The current is a measure of the rate of the electrochemical reaction, which is why voltammetry is often described as an "active" technique that consumes a small amount of analyte [24] [23].
Experimental Protocols and Applications in Drug Development
Cyclic Voltammetry Protocol for Trace Drug Detection Voltammetry requires a three-electrode system: a Working Electrode (WE) where the reaction of interest occurs, a Reference Electrode (RE) to maintain a known potential, and a Counter Electrode (CE) to complete the circuit [1]. This configuration provides precise control over the working electrode potential.
- Electrode Preparation and Modification: Common working electrodes include glassy carbon (GCE), carbon paste (CPE), and screen-printed carbon electrodes (SPCEs) [22]. To enhance sensitivity and selectivity for specific drug targets, the electrode surface is often modified. This can involve drop-casting a suspension of nanomaterials (e.g., graphene, carbon nanotubes, MXenes, or metal nanoparticles) to increase surface area and electron transfer kinetics [22]. The modified electrode is then thoroughly rinsed and dried.
- Measurement Protocol: The three-electrode system is immersed in an electrolyte solution containing the analyte. For cyclic voltammetry, the potential is swept linearly between two set limits at a defined scan rate (e.g., 0.01 to 1 V/s) while the current is recorded. Multiple cycles may be run to assess electrode stability and reaction reversibility [1] [22].
- Data Analysis: The voltammogram is analyzed for peak potentials (for qualitative identification) and peak currents (for quantitative analysis). A plot of (i_p) vs. (\nu^{1/2}) should yield a straight line for a diffusion-controlled process, validating the application of the Randles-Ševčík equation. Quantitative analysis is performed using a calibration curve of peak current versus analyte concentration.
Voltammetry's ability to provide both qualitative and quantitative data makes it a preferred method for a wide range of pharmaceutical applications, from quantifying heavy metals in drug precursors to analyzing the concentration of a new drug compound like antibiotics or nonsteroidal anti-inflammatory drugs (NSAIDs) [1] [22]. Advanced pulsed techniques such as Differential Pulse Voltammetry (DPV) and Square Wave Voltammetry (SWV) offer even higher sensitivity for trace analysis by minimizing background (charging) current [1] [22].
Comparative Analysis: Measurement Paradigms and Analytical Figures of Merit
The core distinction between these techniques lies in their fundamental measurement approach: potentiometry measures potential at zero current (a thermodynamic equilibrium measurement), while voltammetry measures current as a function of applied potential (a kinetic measurement involving analyte consumption) [24]. This fundamental difference dictates their respective applications, advantages, and limitations in drug research.
Table 1: Comparative analysis of potentiometry and voltammetry core characteristics.
| Feature | Potentiometry | Voltammetry |
|---|---|---|
| Governing Equation | Nernst Equation | Randles-Ševčík Equation |
| Measured Signal | Potential (V) | Current (A) |
| Current Flow | Virtually zero [24] | Measured and controlled |
| Analyte Consumption | Negligible [24] | Measurable, though small [24] |
| Primary Application | Ion activity (pH, Na⁺, K⁺) [1] | Redox-active species (drugs, metals) [1] |
| Key Advantage | Non-destructive; ideal for small volumes [24] | High sensitivity; qualitative & quantitative data [1] |
| Key Challenge | Achieving high selectivity with ionophores [24] | Mass transport limitations in small volumes [24] |
Table 2: Analytical performance and typical applications in pharmaceutical sciences.
| Parameter | Potentiometry | Voltammetry |
|---|---|---|
| Detection Limit | ~10⁻⁸ M (varies with ISE) [24] | Can reach 10⁻⁹ M or lower with modified electrodes [22] [24] |
| Selectivity | Achieved via ionophore in membrane [1] | Achieved via potential control & surface modification [22] |
| Sample Volume | Suitable for very small volumes (e.g., 200 µL) [24] | Microelectrodes enable work in small volumes [24] |
| Pharma Application Example | Electrolyte analysis in clinical formulations [1] | Detection of antibiotics, NSAIDs in bio-fluids [22] |
Essential Research Reagents and Materials
The experimental implementation of these electrochemical techniques relies on a standardized set of reagents and materials.
Table 3: Key research reagent solutions and materials for electrochemical analysis.
| Reagent/Material | Function/Description | Typical Examples |
|---|---|---|
| Reference Electrode | Provides a stable, known reference potential for measurements. | Saturated Calomel Electrode (SCE), Ag/AgCl electrode [1] [23]. |
| Working Electrode | The electrode where the controlled reaction occurs. | Glassy Carbon (GCE), Gold, Platinum, Screen-Printed Electrodes (SPEs) [22] [23]. |
| Counter/Auxiliary Electrode | Completes the electrical circuit, carrying the current. | Platinum wire [1] [23]. |
| Supporting Electrolyte | Carries current and minimizes migration; sets ionic strength. | Phosphate buffer, KCl, NaClO₄ [22]. |
| Ionophore | A host molecule that selectively binds a target ion in ISE membranes. | Dicyclohexyl-18-crown-6 (for cations), valinomycin (for K⁺) [24]. |
| Membrane Components (ISE) | Form the ion-selective membrane. | PVC (polymer matrix), oNPOE (plasticizer), KTpClPB (ion exchanger) [24]. |
| Nanomaterials | Modify electrode surfaces to enhance sensitivity and selectivity. | Graphene, Carbon Nanotubes, Metal Nanoparticles, MXenes [22]. |
Experimental Workflow and Signaling Pathways
The following diagrams illustrate the fundamental operational and signaling pathways for potentiometric and voltammetric measurements.
The Nernst and Randles-Ševčík equations govern two distinct yet complementary electrochemical universes: the thermodynamic world of equilibrium potential and the kinetic world of faradaic current. For researchers in drug development, the choice between potentiometric and voltammetric methods hinges on the specific analytical problem. Potentiometry, with its minimal analyte consumption, is ideal for direct ion activity measurement where suitable selective membranes exist. Voltammetry, with its superior sensitivity and rich mechanistic information, is unparalleled for detecting redox-active pharmaceutical compounds at trace levels in complex matrices.
Future trends point toward the miniaturization of these platforms into portable, paper-based analytical devices [25] and the integration of advanced materials like MXenes [22] and quantum principles [26] to push the boundaries of sensitivity and selectivity. The ongoing convergence of these techniques with automation and machine learning [27] promises to further solidify electrochemical analysis as an indispensable tool in the drug development pipeline.
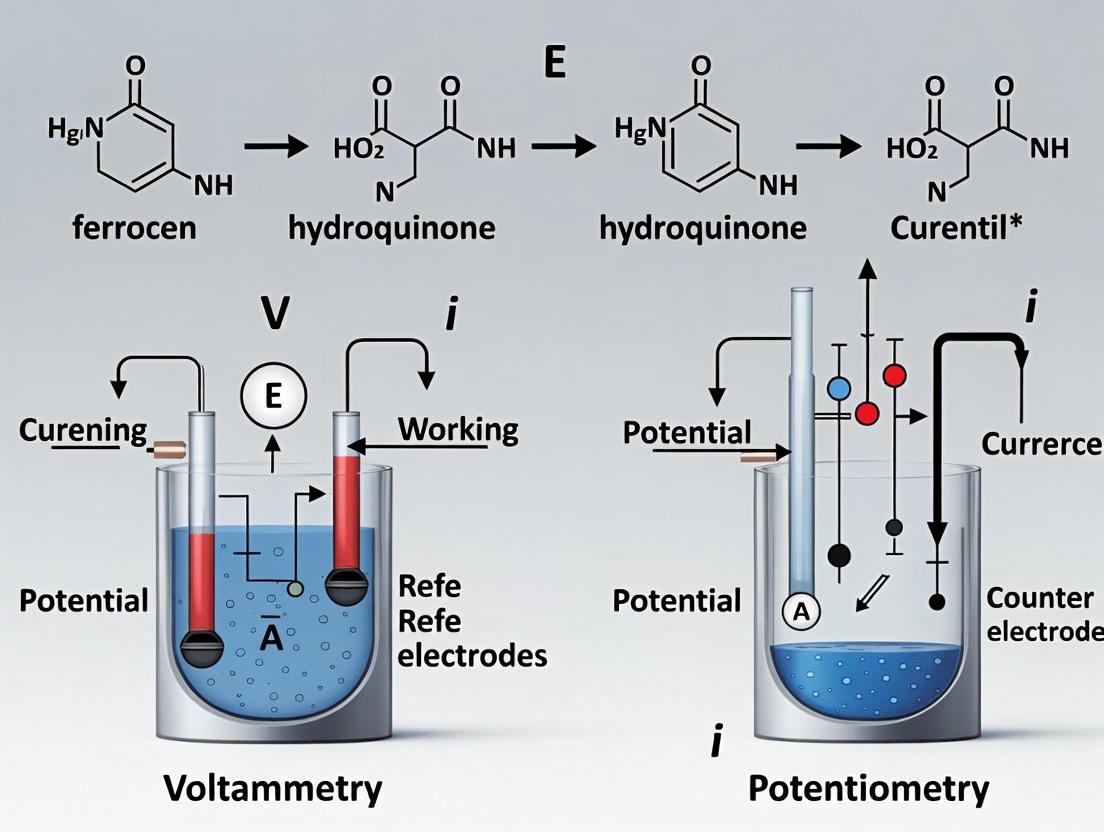
Electrochemical analysis techniques are fundamental tools in modern research, enabling the characterization of redox processes, material properties, and chemical concentrations. These techniques are broadly categorized based on whether they measure current or potential, which dictates their experimental setup and application. Voltammetry is a class of techniques that involves measuring the current response of an electrochemical system while varying an applied potential. In contrast, potentiometry is a technique that involves measuring the potential difference between two electrodes under conditions of zero or negligible current flow [28] [29]. This fundamental distinction—measuring current under applied potential versus measuring equilibrium potential—is the cornerstone upon which their respective electrode systems are built. The selection between a three-electrode voltammetric cell and a two-electrode potentiometric cell is therefore determined by the very nature of the electrochemical information sought.
This guide details the two primary electrode systems: the three-electrode setup essential for voltammetric techniques like Cyclic Voltammetry (CV), and the two-electrode cell utilizing Ion-Selective Electrodes (ISEs) for potentiometric measurements. The content is framed within a broader thesis on analytical measurement, contrasting the dynamic current monitoring of voltammetry with the equilibrium potential measurement of potentiometry.
Three-Electrode Systems for Voltammetry
Principles and Configuration
Voltammetry encompasses techniques where the current at a working electrode is measured as the applied potential is systematically varied [30] [29]. This process drives redox reactions, and the resulting current provides information on reaction kinetics, thermodynamics, and analyte concentration. The three-electrode system is critical for these measurements because it separates the functions of potential control and current carrying [31].
A typical three-electrode system consists of:
- Working Electrode (WE): This is the electrode where the reaction of interest occurs. Its potential is controlled and measured relative to the reference electrode. Common materials include glassy carbon, platinum, and gold [31].
- Counter Electrode (CE) / Auxiliary Electrode: This electrode completes the electrical circuit, allowing current to flow through the cell. It is typically made from an inert material like platinum or graphite and has a large surface area to ensure it does not become a limiting factor in the measurement [30] [31].
- Reference Electrode (RE): This electrode provides a stable, known, and constant reference potential against which the working electrode's potential is both controlled and measured. It is designed so that minimal current passes through it, preserving its stable potential. Common examples include the Ag/AgCl electrode and the Saturated Calomel Electrode (SCE) [31] [32].
The system operates via a potentiostat, an electronic instrument that creates two distinct circuits: a potential circuit between the WE and RE for accurate potential control, and a current circuit between the WE and CE for current measurement [31]. This separation is vital because if significant current were to pass through the reference electrode, its potential would drift due to polarization, leading to inaccurate measurements [33].
Experimental Protocol: Cyclic Voltammetry
Cyclic Voltammetry (CV) is a powerful and widely used voltammetric technique for studying the redox properties of electroactive species [30] [29].
Methodology:
- Cell Assembly: Prepare an electrochemical cell containing the electrolyte and analyte solution. Insert the three electrodes: Working Electrode, Counter Electrode, and Reference Electrode. Ensure the RE is positioned close to the WE to minimize uncompensated solution resistance [31].
- Instrument Setup: Connect the electrodes to a potentiostat. Set the initial potential, upper potential limit, lower potential limit, and the scan rate (e.g., 0.1 V/s). The potential is swept linearly from the initial potential to the upper limit.
- Scan Reversal: Upon reaching the upper potential limit, the scan direction is reversed, and the potential is swept back to the lower limit. This cycle may be repeated multiple times [30].
- Data Collection: The potentiostat measures the current flowing at the working electrode as a function of the applied potential. The result is a cyclic voltammogram—a plot of current (I) vs. potential (E) [30].
Data Interpretation: A typical CV for a reversible redox couple displays a "duck-shaped" plot. Key features include [30]:
- Anodic Peak Current (ipa) and Potential (Epa): Correspond to the oxidation half-reaction.
- Cathodic Peak Current (ipc) and Potential (Epc): Correspond to the reduction half-reaction.
- For a reversible, diffusion-controlled system, the peak currents are equal in magnitude but opposite in sign (|ipa| = |ipc|), and the peak separation (ΔEp = Epa - Epc) is approximately 59 mV for a one-electron transfer process.
The peak current is quantitatively described by the Randles-Ševčík equation (at 298 K): [ ip = (2.69 \times 10^5) \cdot n^{3/2} \cdot A \cdot D^{1/2} \cdot C \cdot v^{1/2} ] where ( ip ) is the peak current (A), ( n ) is the number of electrons transferred, ( A ) is the electrode area (cm²), ( D ) is the diffusion coefficient (cm²/s), ( C ) is the concentration (mol/cm³), and ( v ) is the scan rate (V/s) [30].
The diagram below illustrates the experimental workflow and current response in a cyclic voltammetry experiment.
Research Reagent Solutions for Voltammetry
Table 1: Key reagents and materials for voltammetry experiments.
| Item | Function/Description | Examples & Notes |
|---|---|---|
| Potentiostat | Electronic instrument that controls the potential between WE and RE and measures current between WE and CE [31]. | Essential for all voltammetric experiments. |
| Working Electrode | Surface where the redox reaction of interest occurs [31]. | Glassy Carbon Electrode (GCE), Platinum (Pt) Electrode, Gold (Au) Electrode. Surface pre-treatment is critical for reproducibility [31]. |
| Reference Electrode | Provides a stable, known reference potential for the working electrode [31] [32]. | Ag/AgCl, Saturated Calomel Electrode (SCE). Must maintain stable composition. |
| Counter Electrode | Conducts current to balance the reaction at the working electrode [30] [31]. | Platinum wire, graphite rod. Should have a large surface area. |
| Supporting Electrolyte | Conducts current and minimizes migration of the analyte via ionic strength adjustment [30]. | Inert salts (e.g., KCl, KNO₃, TBAPF₆) at concentrations typically >0.1 M. |
| Redox Active Analyte | The chemical species under investigation. | e.g., Ferrocene, often used as an internal standard [30]. |
| Solvent | Dissolves the electrolyte and analyte. | Water, Acetonitrile (MeCN), Dichloromethane (DCM). Must be pure and electrochemically inert in the potential window of interest. |
Ion-Selective Electrodes for Potentiometry
Principles and Configuration
Potentiometry is an electrochemical technique where the potential (electromotive force, EMF) between two electrodes is measured under conditions of zero or negligible current flow [7] [29]. This measured potential is related to the activity (concentration) of a specific ion in solution via the Nernst equation. The core sensor in this technique is the Ion-Selective Electrode (ISE) [34] [32].
A typical potentiometric cell requires only two electrodes [28]:
- Ion-Selective Electrode (ISE): This is the sensing electrode. It incorporates a specialized membrane that selectively interacts with the target ion, generating a membrane potential that depends on the ionic activity. The key component is the ion-selective membrane, which can be glass, crystalline, or a polymer-based ion-exchange resin [32] [35].
- Reference Electrode: This electrode maintains a constant and known potential, independent of the sample solution's composition. It completes the electrochemical cell and provides a stable reference point for the measurement [32] [35].
The fundamental principle is described by the Nernst equation, which relates the measured cell potential (E) to the activity of the target ion (aion): [ E = E^0 \pm \frac{2.303RT}{zF} \log(a{ion}) ] where E⁰ is the standard cell potential, R is the gas constant, T is temperature, z is the ion's charge, and F is the Faraday constant [28] [35]. The sign is positive for cations and negative for anions. The term (2.303RT/zF) is the Nernstian slope; for a monovalent ion (z=1) at 25°C, it is 59.16 mV per decade change in activity [35].
Experimental Protocol: Direct Potentiometry with an ISE
Direct potentiometry is a straightforward method for determining the concentration of an ion in a sample solution.
Methodology:
- Calibration:
- Prepare a series of standard solutions with known concentrations of the target ion, incorporating an Ionic Strength Adjustment Buffer (ISAB) to maintain a constant background ionic strength. This ensures that activity coefficients are constant, allowing concentration to be used in place of activity [35].
- Immerse the ISE and reference electrode in each standard solution, starting with the most dilute.
- Measure the stable potential (EMF) for each standard.
- Plot the measured EMF (mV) versus the logarithm of the ion concentration (log C). The plot should be linear, conforming to the Nernst equation [35].
- Sample Measurement:
- Immerse the cleaned ISE and reference electrode into the unknown sample solution, which should also contain the same ISAB.
- Measure the stable potential (EMF).
- Determine the unknown concentration from the calibration curve using the measured EMF value.
Data Interpretation: The calibration curve's linear range defines the usable concentration range for the ISE. The detection limit is typically the concentration at which the calibration curve significantly deviates from linearity [35]. The slope of the calibration curve should be close to the theoretical Nernstian value for ideal behavior. A real calibration curve may show sub-Nernstian response at very low concentrations [35].
The diagram below illustrates the structure and operating principle of a solid-contact ion-selective electrode (SC-ISE), a common modern configuration.
Research Reagent Solutions for Potentiometry
Table 2: Key reagents and materials for potentiometry with Ion-Selective Electrodes.
| Item | Function/Description | Examples & Notes |
|---|---|---|
| Ion-Selective Electrode (ISE) | The sensing electrode with a membrane selective for a specific ion [32]. | pH glass electrode, Fluoride ISE (LaF₃ crystal), Potassium ISE (Valinomycin/PVC membrane). |
| Reference Electrode | Provides a stable reference potential against which the ISE potential is measured [32] [35]. | Ag/AgCl with fixed KCl filling solution. Junction potential must be stable. |
| Ionic Strength Adjustment Buffer (ISAB) | Added to standards and samples to maintain constant ionic strength, fix pH, and mask interfering ions [35]. | Critical for accurate measurement; composition depends on analyte and sample matrix. |
| Standard Solutions | Solutions of known concentration for calibrating the ISE [35]. | Should bracket the expected unknown concentration; prepared with high-purity reagents. |
| High-Impedance Potentiometer / pH Meter | Measures the potential difference between the ISE and reference electrode [34] [35]. | Requires high input impedance (>10¹² Ω) to prevent current draw and electrode polarization [34]. |
Core Functional Distinctions
Table 3: A direct comparison of the three-electrode voltammetry system and the two-electrode potentiometry system.
| Feature | Three-Electrode System (Voltammetry) | Ion-Selective Electrode (Potentiometry) |
|---|---|---|
| Primary Measurement | Current (i) as a function of applied potential [29]. | Potential (EMF) at zero current [7] [29]. |
| Key Relationship | Current ∝ Rate of redox reaction & analyte concentration [30]. | Potential ∝ log(Ion Activity) via Nernst equation [28] [35]. |
| Electrode Configuration | Working, Counter, and Reference Electrodes [31]. | Ion-Selective Electrode and Reference Electrode [32] [35]. |
| System State | Dynamic (non-equilibrium); potential is actively scanned [30]. | Static (equilibrium); potential is measured at steady-state [7]. |
| Information Obtained | Redox potentials, reaction kinetics, diffusion coefficients, electron transfer mechanisms [30] [29]. | Ionic activity (concentration) of a specific ion [34] [32]. |
| Key Instrument | Potentiostat [30] [31]. | High-impedance Voltmeter / pH Meter [34] [35]. |
| Data Output | Cyclic Voltammogram (I vs. E plot) [30]. | Calibration curve (EMF vs. log C) and single EMF reading [35]. |
Advanced Trends and Research Impact
The fields of voltammetry and potentiometry continue to evolve, driven by advancements in materials science and manufacturing:
Voltammetry: Recent progress focuses on using microelectrodes and nanoelectrodes for enhanced spatial resolution and sensitivity, and the development of novel electrode materials like carbon nanomaterials to improve electrocatalytic properties and detection limits [29]. Integration with other techniques, such as spectroscopy, is also a growing trend for studying complex systems [29].
Potentiometry: The most significant recent trends involve the move toward solid-contact ISEs (SC-ISEs), which eliminate the internal filling solution of traditional ISEs. This improves mechanical stability, enables miniaturization, and allows for longer sensor lifetime [7]. Key developments in this area include:
- Novel Transducer Materials: Use of conducting polymers (e.g., PEDOT), carbon nanotubes, graphene, and MXenes as the solid-contact layer to enhance capacitance and signal stability [7].
- 3D Printing: Allows for rapid prototyping and fabrication of ISEs with complex geometries, accelerating sensor optimization and development [7].
- Wearable Sensors: The miniaturization and solid-contact design enable the integration of ISEs into wearable devices for continuous monitoring of electrolytes (e.g., K⁺, Na⁺) and drugs in biological fluids like sweat [7].
These advancements are particularly impactful for the target audience of researchers and drug development professionals. Voltammetry is indispensable for characterizing redox-active drug molecules, studying electron transfer mechanisms in biological systems, and developing biosensors. Potentiometry, especially with the advent of miniaturized and wearable SC-ISEs, offers powerful tools for therapeutic drug monitoring (TDM) of pharmaceuticals with narrow therapeutic indices and for real-time tracking of critical electrolytes in clinical settings [7].
Techniques in Action: Applying Voltammetry and Potentiometry in Drug Development
Electroanalytical techniques have emerged as powerful tools in the pharmaceutical industry, offering distinct advantages for drug development, quality assurance, and therapeutic monitoring. Unlike traditional methods such as chromatography and spectrophotometry, electroanalysis provides high sensitivity, rapid analysis, cost-effectiveness, and minimal sample preparation requirements [36]. Among these techniques, voltammetry represents a particularly versatile family of methods that measure current as a function of applied potential to obtain both qualitative and quantitative information about electroactive species [1]. This stands in direct contrast to potentiometry, which measures potential at zero current and is primarily used for ion activity measurements [7] [1].
The fundamental principle of voltammetry involves applying a controlled potential to an electrochemical cell containing a working electrode, reference electrode, and counter electrode, then measuring the resulting current generated by redox reactions at the working electrode interface [1]. This current response provides a wealth of information about the analyte, including its concentration, redox properties, and reaction kinetics. For pharmaceutical researchers and drug development professionals, voltammetric techniques offer unparalleled capabilities for detecting active pharmaceutical ingredients (APIs), monitoring drug metabolites in biological fluids, ensuring product stability, and screening for impurities [36].
This technical guide provides an in-depth examination of three cornerstone voltammetric techniques—cyclic voltammetry (CV), differential pulse voltammetry (DPV), and square wave voltammetry (SWV)—with a specific focus on their application to pharmaceutical analysis. The content is structured to serve as both a foundational reference and a practical resource for implementing these methods in research and quality control environments.
Fundamental Principles: Current Measurement in Voltammetry Versus Potential Measurement in Potentiometry
Comparative Theoretical Foundations
Understanding the distinction between voltammetry and potentiometry begins with recognizing their fundamental measurement approaches. Voltammetry is a dynamic technique that applies a controlled, changing potential to drive redox reactions while measuring the resulting faradaic current.- This current is directly proportional to the concentration of the electroactive species and provides information about reaction kinetics and mechanisms [1]. In contrast, potentiometry is a zero-current technique that measures the equilibrium potential across an interface, relating this potential to analyte concentration through the Nernst equation without net electrochemical reaction occurring [7] [1].
The practical implications of this distinction are significant for pharmaceutical analysis. Voltammetry's current measurement enables exceptional sensitivity, with detection limits often reaching nanomolar or even picomolar concentrations, making it ideal for trace analysis of drugs and metabolites [36]. Potentiometry, while excellent for continuous monitoring of ions like sodium, potassium, and calcium in clinical settings, typically offers higher detection limits and is primarily limited to ionic species [7] [1].
Instrumentation and Cell Configuration
Both voltammetry and potentiometry employ electrochemical cells with working, reference, and counter electrodes. However, voltammetry requires precise potential control and current measurement capabilities provided by modern potentiostats [37]. The working electrode material (glassy carbon, platinum, mercury, or modified electrodes) significantly influences sensitivity and selectivity in voltammetric analysis, while potentiometric systems rely primarily on ion-selective membranes with specific recognition elements [7] [1].
Table 1: Core Differences Between Voltammetry and Potentiometry
| Parameter | Voltammetry | Potentiometry |
|---|---|---|
| Measured Signal | Current | Potential |
| Applied Signal | Variable potential | Zero current |
| Detection Limits | Nanomolar to picomolar | Millimolar to micromolar |
| Primary Applications | Trace drug analysis, metabolite monitoring, reaction mechanism studies | Ion activity measurement (Na+, K+, Ca2+), continuous monitoring |
| Information Obtained | Concentration, kinetics, reaction mechanisms | Ion activity/centration |
| Technique Variants | CV, DPV, SWV, NPV | Direct potentiometry, potentiometric titration |
Cyclic Voltammetry (CV): Fundamentals and Experimental Protocol
Theoretical Principles and Pharmaceutical Applications
Cyclic voltammetry is the most widely used voltammetric technique for initial electrochemical characterization of pharmaceutical compounds. In CV, the potential is scanned linearly from an initial value to a switching potential, then reversed back to the starting potential at a controlled scan rate [1]. The resulting current-potential plot (voltammogram) provides characteristic "peaks" corresponding to oxidation and reduction processes, yielding crucial information about redox potentials, reaction reversibility, electron transfer kinetics, and coupled chemical reactions [36].
For pharmaceutical analysis, CV serves as an indispensable tool for investigating the electrochemical behavior of new drug entities, studying metabolic pathways, and understanding degradation mechanisms [36]. The technique provides qualitative "fingerprints" of redox processes that help researchers predict stability, understand metabolic transformations, and design electroanalytical methods for quantification.
Experimental Protocol for Drug Compound Characterization
Equipment and Reagents:
- Potentiostat with cyclic voltammetry capability
- Three-electrode system: Working electrode (glassy carbon, platinum, or modified electrode), reference electrode (Ag/AgCl or SCE), counter electrode (platinum wire)
- Electrolyte solution (e.g., 0.04 M Britton-Robinson buffer, pH 2.0-12.0) [38]
- Nitrogen gas for deaeration
- Drug compound standard solution
Procedure:
- Prepare supporting electrolyte solution appropriate for the drug's solubility and electrochemical properties. Common choices include Britton-Robinson buffer for wide pH range studies or phosphate buffer for physiological pH simulations [38].
- Polish the working electrode with alumina slurry (0.05 μm) on a microcloth pad, rinse thoroughly with deionized water, and dry.
- Transfer 10-15 mL of supporting electrolyte to the electrochemical cell and deaerate with nitrogen for 8-10 minutes to remove dissolved oxygen.
- Immerse the three-electrode system in the solution and initiate a blank CV scan to establish baseline electrode behavior.
- Add known aliquots of drug standard solution to the cell using a micropipette, mixing between additions.
- Record cyclic voltammograms across the relevant potential window at scan rates typically ranging from 20-500 mV/s.
- Analyze peak current versus scan rate relationships to determine whether processes are diffusion-controlled or adsorption-controlled.
- Calculate redox potentials (Epa, Epc), peak separation (ΔEp), and peak current ratios (Ipa/Ipc) to assess reaction reversibility.
Data Interpretation: Reversible systems display peak separation (ΔEp) of approximately 59/n mV, with peak current ratio near unity. Quasireversible and irreversible processes show larger peak separations and unequal peak currents. The relationship between peak current and square root of scan rate indicates diffusion control, while direct proportionality to scan rate suggests adsorption control [36].
Differential Pulse Voltammetry (DPV): Fundamentals and Experimental Protocol
Theoretical Principles and Pharmaceutical Applications
Differential pulse voltammetry is a highly sensitive pulse technique that effectively minimizes non-faradaic (charging) current, enabling significantly lower detection limits compared to CV [37]. In DPV, a series of small amplitude potential pulses (typically 10-100 mV) is superimposed on a linear staircase potential ramp. Current is sampled twice per pulse—just before pulse application and at the end of the pulse—with the difference between these measurements plotted against the base potential [37]. This differential current measurement cancels most capacitive background current, dramatically improving signal-to-noise ratio for trace analysis [1] [37].
DPV has proven exceptionally valuable in pharmaceutical analysis for quantifying drugs in complex matrices like serum, urine, and pharmaceutical formulations [39] [40]. Its high sensitivity and minimal interference make it ideal for therapeutic drug monitoring, pharmacokinetic studies, and quality control of low-dose formulations.
Experimental Protocol for Trace Drug Quantification
Equipment and Reagents:
- Potentiostat with DPV capability (e.g., Gamry Instruments with PV220 Pulse Voltammetry Software) [37]
- Three-electrode system appropriate for analysis (HMDE for reducible compounds, solid electrodes for oxidizable compounds)
- Supporting electrolyte optimized for target drug (e.g., Clark-Lubs buffer for zalcitabine) [40]
- Standard drug solutions and quality control samples
Procedure:
- Select optimal supporting electrolyte and pH based on preliminary CV studies or literature data. For zalcitabine analysis, Clark-Lubs buffer (pH 2.0) provided maximal response [40].
- Prepare electrode system. For hanging mercury drop electrode (HMDE), dispense a fresh drop for each measurement.
- Transfer 10 mL of supporting electrolyte to the electrochemical cell and deaerate with nitrogen for 5-8 minutes.
- Set DPV parameters based on compound characteristics:
- Record background voltammogram in pure supporting electrolyte.
- Add known aliquots of standard drug solution, recording DPV after each addition.
- Construct calibration curve by plotting peak current versus concentration.
- For formulation analysis, prepare samples by dissolving tablets/serum in appropriate solvent, filtering if necessary, and diluting to working range [39].
Validation Parameters:
- Linearity: Typically 1-50 μg/mL depending on compound [39] [40]
- Detection limit: As low as 2.08 mg/L demonstrated for zalcitabine [40]
- Precision: Intra-day and inter-day RSD <5%
- Accuracy: Recovery of 95-105% from spiked samples
Table 2: Comparison of Key Voltammetric Techniques for Pharmaceutical Analysis
| Parameter | Cyclic Voltammetry (CV) | Differential Pulse Voltammetry (DPV) | Square Wave Voltammetry (SWV) |
|---|---|---|---|
| Primary Use | Mechanism study, redox characterization | Trace quantification | Ultra-sensitive detection, rapid analysis |
| Detection Limit | Micromolar | Nanomolar | Sub-nanomolar |
| Scan Rate | Variable (20-1000 mV/s) | Slow to moderate (10-20 mV/s) | Very fast (effective rates >1 V/s) |
| Background Suppression | Poor | Excellent | Excellent |
| Analysis Time | Moderate | Slow | Very fast |
| Pharmaceutical Example | Redox mechanism of alkaloids [41] | Zalcitabine in medications [40] | Diclofenac in serum [39] |
| Key Advantage | Rich mechanistic information | High sensitivity for irreversibile systems | Speed and sensitivity for reversible systems |
Square Wave Voltammetry (SWV): Fundamentals and Experimental Protocol
Theoretical Principles and Pharmaceutical Applications
Square wave voltammetry is arguably the most sensitive and rapid pulsed voltammetric technique, making it particularly suitable for high-throughput pharmaceutical analysis [42]. In SWV, a symmetrical square wave is superimposed on a staircase potential ramp, with current sampled at the end of each forward and reverse pulse. The net current (difference between forward and reverse currents) is plotted against the base potential, effectively eliminating capacitive background and providing significantly enhanced sensitivity [42]. Key advantages include insensitivity to dissolved oxygen (eliminating deaeration requirements in many cases), extremely fast scan times, and compatibility with advanced electrode materials [42].
SWV has been successfully applied to pharmaceutical analysis for compounds including diclofenac in serum [39], bumadizone in pharmaceutical formulations [38], and various alkaloids [41]. Its robustness in complex biological matrices makes it invaluable for therapeutic drug monitoring and clinical pharmacology studies.
Experimental Protocol for Ultra-Sensitive Drug Detection
Equipment and Reagents:
- Modern potentiostat with SWV capability
- Advanced working electrodes (nano-reduced graphene oxide, carbon paste, or modified electrodes) [38]
- Britton-Robinson buffer for wide pH optimization studies
- Drug standard and biological samples (serum, urine)
Procedure:
- Select and prepare working electrode. For bumadizone analysis, 10% nRGO-modified electrode showed optimal performance [38].
- Optimize supporting electrolyte composition and pH. For diclofenac analysis, 0.1 M TBAClO4/acetonitrile solution enabled detection at platinum electrode [39].
- Set SWV parameters based on preliminary studies:
- Square wave amplitude: 25-50 mV
- Step potential: 2-10 mV
- Frequency: 15-25 Hz
- Accumulation potential and time (if adsorption is employed)
- For biological samples, implement simple protein precipitation (e.g., with ZnSO4/methanol) followed by centrifugation and dilution with supporting electrolyte [39].
- Record square wave voltammograms, identifying characteristic oxidation/reduction peaks.
- Construct calibration curves using standard additions method for matrix-matched samples.
- Validate method according to ICH guidelines including specificity, linearity, accuracy, precision, and robustness [38].
Performance Characteristics:
- Linear range: 1.5-17.5 μg/mL demonstrated for diclofenac [39]
- Detection limits: As low as 0.9×10² to 15×10² ng/mL for bumadizone using nRGO electrodes [38]
- Recovery: >95% from spiked serum samples with minimal interference [39]
Advanced Applications and Future Perspectives
Nanomaterial-Modified Electrodes in Pharmaceutical Voltammetry
The integration of nanotechnology has dramatically enhanced the capabilities of voltammetric techniques in pharmaceutical analysis [36]. Nanostructured electrodes, particularly those incorporating graphene, carbon nanotubes, metal nanoparticles, and their composites, offer increased electroactive surface area, enhanced electron transfer kinetics, and improved antifouling properties [36] [38]. For instance, nano-reduced graphene oxide (nRGO) electrodes demonstrated superior performance for bumadizone determination compared to conventional carbon paste electrodes, enabling nanogram-level detection limits [38]. These advanced materials address traditional challenges in pharmaceutical voltammetry, including electrode fouling from complex matrices and insufficient sensitivity for ultratrace analysis.
Analysis in Complex Matrices: From Formulations to Biological Fluids
Voltammetric techniques have proven remarkably adaptable to challenging analytical scenarios involving complex sample matrices. SWV methods have been successfully validated for direct determination of diclofenac in human serum without interference from endogenous compounds [39]. Similarly, DPV has enabled zalcitabine quantification in pharmaceutical dosage forms with minimal sample preparation [40]. The key to success in these applications lies in careful optimization of supporting electrolyte, pH, electrode material, and potential waveform parameters to maximize selectivity for the target analyte while minimizing matrix effects.
Emerging Trends: Miniaturization, Automation, and Green Analytical Chemistry
The future trajectory of voltammetric pharmaceutical analysis points toward increased miniaturization, automation, and alignment with green analytical principles [36]. Lab-on-a-chip devices integrating microfluidic sample handling with miniaturized electrodes promise point-of-care therapeutic drug monitoring capabilities [36]. Meanwhile, the adoption of green assessment tools like AGREE and Eco-scale metrics demonstrates the field's commitment to environmentally sustainable methodology [38]. The convergence of artificial intelligence for experimental optimization and data interpretation with advanced sensor technology positions voltammetry for continued growth as a mainstay of pharmaceutical analysis.
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 3: Key Research Reagents and Materials for Voltammetric Pharmaceutical Analysis
| Reagent/Material | Function/Application | Example Use Cases |
|---|---|---|
| Britton-Robinson Buffer | Wide pH range (2-12) supporting electrolyte | Initial method development, pH optimization studies [38] |
| Clark-Lubs Buffer | Acidic pH electrolyte for reduction studies | Zalcitabine determination at pH 2.0 [40] |
| Nano-Reduced Graphene Oxide (nRGO) | Electrode modifier for enhanced sensitivity | Bumadizone detection at nanogram levels [38] |
| Sodium Dodecyl Sulfate (SDS) | Surfactant for modifying electrode-solution interface | Improving voltammetric response of hydrophobic drugs [38] |
| Tetrahydrofuran/Acetonitrile | Organic solvent for hydrophobic drug dissolution | Diclofenac analysis in non-aqueous medium [39] |
| Paraffin Oil/Graphite Powder | Components for carbon paste electrode preparation | Fabrication of customizable working electrodes [38] |
Experimental Workflow and Signaling Pathways
The following diagram illustrates the logical decision process for selecting and applying appropriate voltammetric techniques in pharmaceutical analysis based on analytical objectives:
Voltammetry Technique Selection Logic
The diagram above outlines the strategic selection process for voltammetric techniques based on analytical requirements. Cyclic voltammetry serves as the starting point for understanding fundamental electrochemical behavior of new pharmaceutical compounds, providing critical data on redox mechanisms and reaction kinetics. When the analytical objective shifts to trace quantification in complex matrices like biological fluids, differential pulse voltammetry offers the necessary sensitivity and selectivity. For applications demanding ultra-sensitive detection with rapid analysis times, square wave voltammetry emerges as the technique of choice, particularly beneficial for high-throughput pharmaceutical quality control and therapeutic drug monitoring.
Electroanalytical techniques are fundamentally divided into methods that measure potential under static conditions and those that measure current under dynamic conditions. Potentiometry, the focus of this guide, involves measuring the potential (electromotive force, emf) of an electrochemical cell under conditions of zero or negligible current flow [7]. This contrasts with voltammetry, where current is measured as a function of an applied potential that drives redox reactions [43]. The core advantage of potentiometry is its minimal disturbance to the sample solution, as the near-zero current means the cell's composition remains essentially unchanged, allowing for direct, rapid readout of ion activities [7] [43]. This makes it a powerful tool for the selective measurement of a wide variety of analytes across clinical, environmental, and industrial applications.
Ion-Selective Electrodes (ISEs): Principles and Components
Ion-Selective Electrodes (ISEs) are membrane-based sensors that convert the activity of a specific ion in solution into an electrical potential [44]. The measured potential is proportional to the logarithm of the ion's activity, as described by the Nernst equation, allowing for sensitive detection over a wide concentration range [44] [43].
Core Components and Function
A typical potentiometric cell using an ISE consists of several key components, whose relationships are outlined in the workflow below.
The potential of the entire cell ((E{cell})) is the difference between the indicator electrode's potential ((E{ise})) and the reference electrode's potential ((E{ref})), with an additional small contribution from the potential drop over the solution ((E{sol})) [45] [46]. This relationship is defined as:
(E{cell} = E{ise} - E{ref} + E{sol}) [45]
The ISE itself is composed of:
- Ion-Selective Membrane: The heart of the ISE, it is permeable only to the target ion, generating a membrane potential [44] [47].
- Internal Filling Solution: A solution containing a fixed activity of the target ion [47].
- Internal Reference Electrode: Typically an Ag/AgCl wire immersed in the internal filling solution, providing a stable electrical connection [44].
Membrane Types and Selectivity
The selectivity of the ISE is determined by the composition of its membrane. The table below summarizes the four primary types of ion-selective membranes.
Table 1: Types of Ion-Selective Electrode Membranes
| Membrane Type | Composition | Primary Selectivity | Key Characteristics & Considerations |
|---|---|---|---|
| Glass Membranes [44] | Silicate or chalcogenide glass | Single-charged cations (e.g., H⁺, Na⁺, Ag⁺); some for double-charged ions (e.g., Cd²⁺, Pb²⁺) | High durability in aggressive media. Subject to alkali error (at high pH) and acidic error (at low pH). |
| Crystalline Membranes [44] | Poly- or monocrystalline substance (e.g., LaF₃ for fluoride) | Ions that can enter the crystal lattice (e.g., F⁻, Cl⁻, Br⁻, I⁻, CN⁻, S²⁻) | Good selectivity; no internal solution required. Selectivity depends on the crystal structure. |
| Ion-Exchange Resin Membranes [44] | Organic polymer membrane with ion-exchange substance | Wide range of single- and multi-atom ions; anionic selectivity available | Most common type. Offers versatility but may have lower physical/chemical durability, especially for anions. |
| Enzyme Electrodes [44] | Enzyme-containing membrane covering a true ISE (e.g., pH electrode) | Substrates of the specific enzyme (e.g., glucose) | Not a true ISE; uses a double-reaction mechanism where the enzyme reaction product is detected by the underlying ISE. |
The mechanism of potential development can occur via ion exchange, where a lipophilic ion in the membrane exchanges with the analyte ion in solution, or ion transport with an ionophore, where a neutral carrier molecule selectively "carries" the ion into the membrane phase [47]. Ionophores, such as valinomycin for potassium ions, are crucial for imparting high selectivity to polymer membrane-based ISEs [47].
Solid-Contact ISEs (SC-ISEs)
Traditional ISEs with an internal filling solution (liquid-contact ISEs) can suffer from mechanical instability and evaporation, complicating miniaturization [7]. Solid-Contact ISEs (SC-ISEs) eliminate the internal solution by replacing it with a solid-contact (SC) layer that acts as an ion-to-electron transducer [7]. This layer converts the ionic signal from the membrane into an electronic signal measured as a potential. Common SC materials include:
- Conducting Polymers: Polyaniline, poly(3-octylthiophene), and poly(3,4-ethylenedioxythiophene) [7].
- Carbon-based Materials & Nanocomposites: Colloid-imprinted mesoporous carbon, MXenes, multi-walled carbon nanotubes, and nanomaterials like Fe₃O₄-filled MoS₂ nanoflowers [7].
SC-ISEs offer advantages for miniaturization, portability, and stability, and are particularly suited for developing wearable sensors and embedded systems [7].
Potentiometric Titration: Principles and Methodology
Potentiometric titration is a technique where the electric potential of an electrochemical cell is monitored as a titrant is added, used to characterize acids, bases, redox agents, and ions that form precipitates or complexes [45] [46]. The primary advantage is that it does not require a chemical indicator and can be used with colored or turbid solutions [7] [46].
Fundamental Setup and Procedure
The setup involves an indicator electrode (which can be an ISE or a redox-active metal like platinum) and a reference electrode (e.g., calomel or silver/silver chloride), both immersed in the analyte solution [45] [46]. The potential difference between them is measured after each addition of titrant. A graph of potential ((E_{cell})) versus titrant volume is plotted, and the endpoint of the titration is identified as the point of maximum slope or the inflection point on this curve [45].
Types of Potentiometric Titration
Table 2: Types of Potentiometric Titrations
| Titration Type | Reaction Involved | Common Indicator Electrode | Example Application |
|---|---|---|---|
| Acid-Base Titration [46] | Neutralization | Glass pH electrode | Determining the concentration of an unknown acid or base. |
| Redox Titration [45] [46] | Electron transfer | Platinum or other inert metal | Titrating halide ions with potassium permanganate [45]. |
| Precipitation Titration [45] [46] | Formation of an insoluble salt | Ion-selective electrode (e.g., Ag for halides) | Titrating mercurous solution with potassium chloride, bromide, or iodide [45]. |
| Complexometric Titration [45] [46] | Formation of a soluble complex | Ion-selective electrode or mercury | Determining metal ions using EDTA as a titrant [45]. |
Advanced Trends and Applications
Emerging Trends in Potentiometric Sensing
Recent research has expanded the capabilities and applications of potentiometric sensors through several key trends:
- 3D Printing: Offers improved flexibility, precision, and rapid prototyping for manufacturing ISEs, decreasing optimization time [7].
- Paper-Based Sensors: Serve as cost-effective and versatile platforms for point-of-care (POC) in-field analysis [7].
- Wearable Sensors: Enable the continuous monitoring of biomarkers, electrolytes, and pharmaceuticals in biological fluids like sweat, paving the way for personalized clinical and biomedical applications [7].
- Voltammetric Ion Sensing: Recent research demonstrates that ISEs can also be operated in voltammetric mode (non-zero current), which can enable multi-analyte detection from a single sensor and improve sensitivity and detection limits [48].
Key Application Areas
Potentiometric methods are employed in a vast array of fields, including:
- Clinical and Biomedical Diagnostics: Monitoring physiological electrolytes (e.g., Na⁺, K⁺, Cl⁻, Ca²⁺) in blood and urine is crucial, as imbalances are linked to higher mortality and morbidity [7] [44]. Therapeutic Drug Monitoring (TDM) of pharmaceuticals with a narrow therapeutic index is another critical application [7].
- Environmental Monitoring: Detection of heavy metals (e.g., Lead, Copper, Mercury) in soil and water, as well as nutrients like nitrate, ammonium, and chloride, is essential for assessing water quality and agricultural impact [7] [44].
- Industrial and Pharmaceutical Quality Control: Determining the concentration of active pharmaceutical ingredients in dosage forms and monitoring industrial processes [7] [44].
- Forensic Analysis: Detecting drugs or toxic substances at crime scenes with minimal sample preparation [7].
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table details key materials and reagents essential for conducting experiments with ion-selective electrodes and potentiometric titrations.
Table 3: Essential Research Reagents and Materials for Potentiometry
| Item | Function / Application | Specific Examples / Notes |
|---|---|---|
| Ion-Selective Membranes [44] [47] | The sensing component that provides selectivity for the target ion. | Glass (for H⁺, Na⁺), LaF₃ crystal (for F⁻), Polymer membranes with ionophores (e.g., Valinomycin for K⁺). |
| Ionophores [47] | Neutral carrier molecules in membranes that selectively bind target ions. | Valinomycin (K⁺ selective), Crown ethers (e.g., for Li⁺). Critical for polymer membrane ISEs. |
| Ion-Exchangers [47] | Lipophilic ions in the membrane that facilitate ion-exchange processes. | Often used in cation- or anion-exchange resin membranes. |
| Internal Filling Solution [44] [47] | Provides a fixed, known activity of the target ion for liquid-contact ISEs. | Aqueous solution of KCl saturated with AgCl, containing salts of the target ion. |
| Reference Electrode [44] [43] [45] | Provides a stable, constant half-cell potential for measurement. | Ag/AgCl electrode, Calomel electrode (Hg/Hg₂Cl₂). Often contains concentrated KCl (e.g., 3.5 M). |
| Solid-Contact (SC) Materials [7] | Replaces internal solution in SC-ISEs; acts as an ion-to-electron transducer. | Conducting polymers (PEDOT, polyaniline), carbon nanomaterials (CNTs, graphene), nanocomposites. |
| Redox Couples (for Voltammetric ISE) [48] | Enables voltammetric operation of ISEs for multi-analyte detection. | Ferrocenemethanol (FcMeOH), Ferrocyanide/Ferricyanide ([Fe(CN)₆]⁴⁻/³⁻). Added to internal solution. |
| Standard Buffer Solutions | For calibration of pH ISEs and reference electrodes. | Aqueous solutions of known, precise pH (e.g., pH 4.01, 7.00, 10.01). |
| Ionic Strength Adjuster (ISA) | Added to samples and standards to maintain constant ionic background, minimizing the junction potential. | High concentration of an inert electrolyte (e.g., KNO₃). |
Experimental Protocols
General Protocol for Calibration and Measurement with an ISE
The following workflow diagrams the standard procedure for calibrating an Ion-Selective Electrode and measuring an unknown sample.
Detailed Methodology:
- Preparation of Standard Solutions: Prepare a series of at least three standard solutions of the target ion, spanning the expected concentration range of the unknown. To all standards and samples, add an Ionic Strength Adjuster (ISA) to ensure constant ionic strength and activity coefficients [44].
- Calibration: After rinsing the electrodes with deionized water, immerse the ISE and reference electrode in each standard solution, starting with the most dilute. Measure and record the stable potential (in millivolts) for each standard.
- Calibration Curve: Plot the measured potential (E) versus the logarithm of the ion activity (log a_i). The plot should yield a straight line. A slope of approximately ±59.2 mV per decade (at 25°C) for a monovalent ion, or ±29.6 mV per decade for a divalent ion, indicates Nernstian behavior and a properly functioning sensor [44] [43].
- Sample Measurement: Rinse the electrodes and measure the potential of the unknown sample under identical conditions. Use the calibration curve to determine the concentration of the unknown.
General Protocol for Potentiometric Titration
Detailed Methodology:
- Setup: Assemble the titration apparatus with the indicator electrode (appropriate for the titration type) and reference electrode immersed in the analyte solution.
- Data Acquisition: Begin magnetic stirring. Add the titrant in small, measured increments. After each addition, wait for the potential to stabilize and then record both the volume of titrant added and the corresponding cell potential.
- Endpoint Determination: Plot the recorded data as potential (E) versus titrant volume (V). The endpoint is the volume at the steepest point of the sigmoidal-shaped curve. The first derivative (ΔE/ΔV vs. V) can be calculated and plotted to pinpoint this volume more precisely.
- Quantification: Use the endpoint volume, the known concentration of the titrant, and the reaction stoichiometry to calculate the concentration of the analyte in the original solution.
Quantifying Active Pharmaceutical Ingredients (APIs) and Metabolites
The accurate quantification of Active Pharmaceutical Ingredients (APIs) and their metabolites is a cornerstone of pharmaceutical development and quality control, ensuring drug safety, efficacy, and stability [49]. Electrochemical methods offer powerful, sensitive, and cost-effective tools for this task. These techniques primarily fall into two categories: those that measure current and those that measure potential. This guide frames the discussion within the core comparison of current measurement in voltammetry versus potential measurement in potentiometry [50] [1]. The choice between these approaches hinges on the specific analytical requirements, including the need for selectivity, sensitivity, the nature of the sample matrix, and the required detection limit.
Voltammetry involves applying a time-dependent potential to an electrochemical cell and measuring the resulting current, which is proportional to the concentration of an electroactive analyte [23]. In contrast, potentiometry measures the potential at an electrode under conditions of zero current, which relates to analyte concentration via the Nernst equation [1]. Understanding the principles, advantages, and limitations of each technique is crucial for developing robust analytical methods for APIs and metabolites.
Core Principles: Voltammetry vs. Potentiometry
Fundamental Concepts and Comparison
The following table summarizes the fundamental differences between these two electrochemical approaches, with a particular focus on the measurement of current in voltammetry and potential in potentiometry.
Table 1: Core Comparison of Voltammetry and Potentiometry
| Feature | Voltammetry | Potentiometry |
|---|---|---|
| Measured Quantity | Current ((i)) [50] [23] | Potential ((E) or (V)) [50] [1] |
| Applied Signal | Time-dependent potential [23] | Zero current (open circuit) [1] |
| Analytical Relationship | Current (\propto) concentration (from Cottrell, etc.) | Potential (\propto \log)(activity) (Nernst equation) [1] |
| Analyte Consumption | Yes, redox reaction consumes analyte [50] | Virtually none [50] |
| Selectivity Mechanism | Applied potential & electrode modification [50] | Ionophore selectivity in membrane [50] [1] |
| Key Advantage | High sensitivity, trace analysis, qualitative & quantitative data [1] | Simple instrumentation, non-destructive, ideal for ions [50] |
| Key Disadvantage | Diffusion limitations, especially in small volumes [50] | Requires highly selective ionophore for specific analytes [50] |
| Example Application | Dopamine detection in nM range [50] | pH measurement, ion-selective electrodes (Na+, K+) [1] |
The Electrochemical Cell and Instrumentation
Most modern electrochemical analyses, particularly voltammetry, use a three-electrode system [1] [23]:
- Working Electrode (WE): Where the redox reaction of interest occurs. Materials include gold, platinum, carbon, or mercury [50] [23].
- Reference Electrode (RE): Provides a stable, known potential (e.g., Ag/AgCl, SCE) against which the WE's potential is measured [1].
- Counter Electrode (Auxiliary Electrode): Completes the circuit, carrying the current needed to balance the current at the WE [1].
This configuration, controlled by a potentiostat, ensures precise control of the WE potential and accurate current measurement [23].
Quantitative Analysis of APIs
Voltammetric Techniques for API Quantification
Voltammetric techniques are highly suited for quantifying electroactive APIs. The current response is directly related to the concentration of the API undergoing oxidation or reduction.
Table 2: Common Voltammetric Techniques for API Analysis
| Technique | Description | Application in Pharma |
|---|---|---|
| Cyclic Voltammetry (CV) | Potential is scanned in a forward and reverse direction [1]. | Studying redox mechanisms, reversibility, and electron transfer kinetics of APIs [1]. |
| Differential Pulse Voltammetry (DPV) | Small potential pulses superimposed on a linear ramp enhance sensitivity [50] [1]. | Trace-level quantification of APIs and metabolites with minimal background current [1]. |
| Square Wave Voltammetry (SWV) | Similar to DPV, but with a square-wave potential, offering even faster and more sensitive analysis [1]. | High-throughput, sensitive detection of drug compounds [1]. |
Experimental Protocol: Voltammetric Sensing of Dopamine
- Objective: To quantify dopamine concentration using a bare gold working electrode.
- Cell Design: A miniature "barrel" type 3-electrode cell is suitable for small sample volumes (~200 µL) [50].
- Electrodes: Gold working electrode, Ag/AgCl reference electrode, platinum counter electrode [50].
- Procedure:
- Clean the gold working electrode surface according to established protocols (e.g., electrochemical cycling in acid) [50].
- Place the sample solution into the electrochemical cell.
- Apply a differential pulse voltammetry (DPV) waveform. A typical sequence might involve potential steps from -0.2 V to +0.5 V vs. Ag/AgCl, with pulse amplitudes of 50 mV.
- Measure the oxidation current peak corresponding to dopamine oxidation.
- Quantification: Construct a calibration curve by plotting the peak current against the concentration of standard dopamine solutions. The detection limit for this method can reach down to 10⁻⁷ M in small volumes [50].
- Key Consideration: The oxidation of dopamine can be diffusion-limited. Stirring or the use of microelectrode arrays can help overcome this limitation and improve sensitivity [50].
Potentiometric Sensing of APIs
Potentiometry offers an alternative for APIs that can be converted into an ionic species. A common approach is the use of Ion-Selective Electrodes (ISEs).
Experimental Protocol: Potentiometric Sensing with an ISE
- Objective: To detect a protonated amine-containing API using a solvent-polymeric membrane ISE.
- Sensor Design: A flow-through tubular unit can be developed for small volumes. The membrane contains [50]:
- Polymer Matrix: e.g., PVC.
- Plasticizer: e.g., 2-nitrophenyloctyl ether (oNPOE).
- Ion Exchanger: e.g., potassium tetrakis(p-Cl-phenyl)borate (KClTPB).
- Ionophore (Optional): A selective crown ether like dicyclohexyl-18-crown-6 (DCH-18-6) to enhance selectivity for the target cation [50].
- Procedure:
- Condition the ISE in a solution containing the target ion.
- Measure the potential difference between the ISE and a reference electrode in a series of standard solutions under zero-current conditions.
- Plot the measured potential vs. the logarithm of the ion activity (concentration).
- Quantification: Use the resulting Nernstian calibration curve ((E) vs. (\log[a])) to determine the concentration in unknown samples. The slope should be close to the theoretical Nernst value (59.2 mV per decade for a monovalent cation at 25°C) [1].
- Key Advantage: Intrinsic selectivity against anions like ascorbic acid and uric acid, which are common interferences in voltammetric bio-sensing [50].
Metabolite Analysis and Cross-Platform Considerations
The quantification of metabolites often involves complex biological matrices, requiring high sensitivity and specificity. While electrochemical methods are applicable, separation techniques coupled to mass spectrometry are widely used.
Inter-laboratory Validation: A major challenge in metabolite measurement is ensuring data consistency across different laboratories and platforms. An inter-laboratory comparison of metabolite measurements revealed that while different methods (e.g., LC-MS, CE-TOFMS) can produce comparable relative quantification data for about half of measured metabolites, issues like erroneous peak identification, insufficient separation, and differences in detection sensitivity can lead to discrepancies [51]. This underscores the need for rigorous method validation and the use of shared reference materials for data normalization [51].
Platform Comparison: A study comparing Ultra-High Performance Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC-HRMS) and Fourier Transform Infrared (FTIR) spectroscopy for serum metabolomics found that UHPLC-HRMS generally yields more robust predictive models for homogeneous populations, providing detailed mechanistic insights. In contrast, FTIR spectroscopy, with its simplicity, speed, and cost-effectiveness, was more suitable for analyzing complex, unbalanced populations, facilitating large-scale studies and clinical translation [52].
Method Validation in Regulatory Context
For any analytical method quantifying APIs or metabolites, validation is mandatory to ensure the results are reliable and suitable for their intended purpose. This process is guided by ICH, FDA, and EMA regulations [53] [54].
Table 3: Key Validation Parameters for Quantitative Analytical Methods
| Parameter | Definition | Application in API/Metabolite Quantification |
|---|---|---|
| Accuracy | Closeness of the measured value to the true value [54]. | Confirms the method accurately quantifies the API in the presence of excipients and impurities [49]. |
| Precision | Degree of agreement among individual test results (Repeatability & Reproducibility) [54]. | Ensures consistent results across multiple analyses of the same homogeneous sample. |
| Specificity | Ability to measure the analyte unequivocally in the presence of other components [54]. | Distinguishes the API from degradation products, impurities, and metabolites [53]. |
| Linearity & Range | The ability to obtain results proportional to analyte concentration, within a given range [54]. | Demonstrates the method is quantitative across the expected concentration levels. |
| LOD & LOQ | Lowest detectable and lowest quantifiable amount of analyte, respectively [54]. | Determines the method's sensitivity for detecting trace impurities or low-dose metabolites. |
| Robustness | Capacity to remain unaffected by small, deliberate variations in method parameters [53]. | Tests the method's resilience to changes in pH, temperature, or mobile phase composition. |
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 4: Key Reagents and Materials for Electrochemical Analysis of APIs
| Item | Function/Brief Explanation |
|---|---|
| Potentiostat | Instrument that controls the potential of the working electrode and measures the resulting current [23]. |
| Three-Electrode Cell | Comprises Working, Reference, and Counter electrodes; the standard setup for precise voltammetry [1]. |
| Gold or Platinum Working Electrode | Inert solid electrodes for oxidation/reduction reactions of electroactive APIs [50]. |
| Ion-Selective Membrane Components | PVC, plasticizer (oNPOE), ion exchanger (KClTPB), and ionophore; form the sensing layer in potentiometric sensors [50]. |
| Standard Reference Materials | Certified materials used for calibration and to normalize data across different laboratories and platforms [51]. |
| HPLC/UHPLC with MS Detector | Provides high separation power and sensitive detection for complex mixtures of APIs and metabolites [52]. |
Workflow and Signaling Visualization
The following diagram illustrates the decision-making workflow for selecting an appropriate analytical technique based on the analytical problem and the nature of the target analyte.
The quantification of APIs and metabolites is critical to modern pharmaceuticals. Voltammetry, with its current-based measurement, offers high sensitivity and is ideal for trace analysis of electroactive species. Potentiometry, with its potential-based measurement, provides a simple, non-destructive route for ionic species, especially when a selective ionophore is available. The choice between these techniques is not a matter of superiority but of appropriateness for the specific analytical challenge. A thorough understanding of their fundamental principles, advantages, and limitations, as framed by the current-potential dichotomy, empowers scientists to develop validated, reliable, and regulatory-compliant methods that ensure drug quality and patient safety.
Therapeutic Drug Monitoring (TDM) and Tracking Electrolytes in Biological Fluids
Therapeutic Drug Monitoring (TDM) and electrolyte tracking represent critical analytical challenges in clinical chemistry and personalized medicine. These applications are primarily advanced through two principal electrochemical sensing frameworks: potentiometry (which measures potential at zero current) and voltammetry (which measures current as a function of applied potential). The fundamental distinction between these approaches lies in their underlying measurement principles—potentiometry provides information about ionic activity or concentration through equilibrium potential measurements, while voltammetry generates information about redox-active species through controlled non-equilibrium current measurements [55] [56]. This technical guide explores how these complementary electrochemical techniques enable precise quantification of pharmaceutical compounds and physiological electrolytes in biological fluids, with a specific focus on their operational principles, methodological considerations, and applications within biomedical research and clinical diagnostics.
The growing importance of TDM is underscored by its role in optimizing dosage regimens for drugs with narrow therapeutic indices, high inter-individual pharmacokinetic variability, or unclear concentration-response relationships [57]. Similarly, monitoring electrolytes such as sodium, potassium, chloride, and calcium is crucial since imbalances can lead to significant neurological, cardiac, and metabolic complications [7]. Electrochemical methods, particularly those incorporating nanomaterials and advanced transducer designs, offer promising alternatives to conventional chromatographic and spectroscopic techniques by providing rapid, cost-effective, and potentially continuous monitoring capabilities suitable for point-of-care testing and personalized medicine applications [58] [57].
Theoretical Foundations: Potentiometry vs. Voltammetry
Fundamental Principles and Measurement Modes
Potentiometry is an electrochemical technique that measures the potential (electromotive force, emf) of an electrochemical cell under static (zero-current) conditions [55] [43]. This potential develops across ion-selective membranes and is related to the activity of target ions through the Nernst equation [43]. In clinical and biomedical applications, potentiometric sensors typically employ ion-selective electrodes (ISEs) coupled with reference electrodes to determine ionic species concentrations in complex biological matrices [7]. A significant advantage of potentiometry includes its insensitivity to electrode size, enabling miniaturization without sacrificing sensitivity [7]. Additionally, the technique exhibits high selectivity toward specific ions, rapid response times, and compatibility with colored or turbid samples [7].
Voltammetry encompasses electrochemical techniques that measure current resulting from applied potential waveforms [55] [56]. Unlike potentiometry, voltammetry operates under non-equilibrium conditions with current flow occurring through redox reactions of electroactive species. The measured current is proportional to analyte concentration and provides information about reaction kinetics, diffusion coefficients, and redox potentials [56]. Voltammetric techniques typically utilize a three-electrode system (working, reference, and counter electrodes) to precisely control potential while accurately measuring current [56]. Advanced voltammetric methods such as anodic stripping voltammetry (ASV) offer exceptional sensitivity for trace metal analysis, while cyclic voltammetry (CV) provides valuable insights into redox mechanisms [56].
Table 1: Core Principles of Potentiometry and Voltammetry
| Feature | Potentiometry | Voltammetry |
|---|---|---|
| Measured Signal | Potential (voltage) | Current |
| Current Flow | Negligible or zero | Significant |
| Fundamental Equation | Nernst equation | Butler-Volmer equation |
| Electrode System | Two-electrode (indicator and reference) | Three-electrode (working, reference, counter) |
| Analytical Relationship | Logarithmic (potential vs. log activity) | Linear (current vs. concentration) |
| Information Obtained | Ionic activity, concentration | Concentration, redox properties, kinetics |
| Detection Limits | Typically ~10⁻⁶ to 10⁻¹¹ M for ions [7] | As low as ~10⁻¹¹ M for stripping methods [56] |
| Key Applications | Electrolytes, pH, ionic drugs | Metals, organic molecules, redox-active drugs |
Sensor Architectures and Transduction Mechanisms
Potentiometric Sensor Designs have evolved from traditional liquid-contact ion-selective electrodes (LC-ISEs) to advanced solid-contact ion-selective electrodes (SC-ISEs) [7]. LC-ISEs incorporate an internal filling solution between the ion-selective membrane and internal reference electrode, but suffer from limitations including mechanical instability, evaporation, and difficulties in miniaturization [7]. SC-ISEs eliminate the inner solution by incorporating a solid-contact layer that functions as an ion-to-electron transducer [7]. These designs offer enhanced miniaturization potential, better stability, and improved performance in complex matrices [7].
Various transducer materials have been investigated for SC-ISEs, including conducting polymers (e.g., poly(3,4-ethylenedioxythiophene), polyaniline, poly(3-octylthiophene)) and carbon-based nanomaterials (e.g., carbon nanotubes, graphene, MXenes) [7]. These materials provide high capacitance and facilitate the conversion between ionic signals in the membrane and electronic signals in the electrode [7]. Recent innovations focus on nanocomposite materials that synergistically enhance sensor performance—for example, MoS₂ nanoflowers filled with Fe₃O₄ nanoparticles increase capacitance and structural stability, while tubular gold nanoparticles with tetrathiafulvalene (Au-TFF) create high-capacitance contacts for potassium sensing [7].
Voltammetric Sensor Configurations have advanced significantly with the development of microelectrodes and nanoelectrodes [56]. While mercury electrodes were historically preferred for their high hydrogen overpotential and amalgam formation properties, concerns about toxicity have shifted research toward alternative materials including glassy carbon, platinum, gold, and carbon paste electrodes [56]. Electrode modification with nanomaterials (e.g., nanoparticles, nanowires, graphene) significantly enhances sensitivity and selectivity by increasing surface area, improving electron transfer kinetics, and enabling functionalization with recognition elements [56].
Analytical Methodologies and Experimental Protocols
Potentiometric Methods for Electrolyte and Drug Monitoring
Potentiometric determination of electrolytes represents a well-established methodology with widespread clinical implementation [7]. The following protocol details a generalized approach for constructing solid-contact ion-selective electrodes for electrolyte monitoring:
Sensor Fabrication Protocol:
- Electrode Pretreatment: Clean the solid substrate (e.g., glassy carbon, gold, or screen-printed electrode) through mechanical polishing and electrochemical activation.
- Solid-Contact Deposition: Apply the transducer layer (e.g., conducting polymer or carbon nanomaterial) via drop-casting, electropolymerization, or spin-coating. Common materials include poly(3,4-ethylenedioxythiophene) doped with poly(styrene sulfonate) (PEDOT:PSS) or colloidal-imprinted mesoporous carbon (C-MSC).
- Ion-Selective Membrane Application: Prepare a membrane cocktail containing ionophore (1-2 wt%), ionic sites (0.5-1 wt%), polymer matrix (e.g., PVC, 30-33 wt%), and plasticizer (65-70 wt%). Deposit the membrane solution onto the solid-contact layer and allow solvent evaporation.
- Conditioning: Soak the prepared sensor in a solution containing the target ion (typically 0.01-0.1 M) for 12-24 hours to establish stable potential response.
- Calibration: Measure the potential response in standard solutions with known activities of the target ion (typically covering 10⁻⁶ to 10⁻¹ M). Plot potential (E) versus log(activity) to obtain the calibration curve.
- Sample Measurement: Immerse the conditioned sensor along with a reference electrode (e.g., Ag/AgCl) in the sample solution and record the potential. Determine concentration from the calibration curve.
For TDM applications using potentiometric sensors, the methodology follows similar principles but employs ionophores with selectivity for specific drug molecules rather than inorganic ions [7]. These sensors have been successfully applied to monitor various pharmaceuticals including antibiotics, antiepileptics, and cardiovascular drugs [7].
Voltammetric Methods for Drug and Metabolite Analysis
Voltammetric techniques offer complementary approaches for monitoring electroactive pharmaceuticals and their metabolites. The following protocol describes a generalized anodic stripping voltammetry method for trace metal analysis relevant to electrolyte monitoring:
Anodic Stripping Voltammetry Protocol:
- Sample Pretreatment: Add supporting electrolyte (e.g., 0.1 M acetate buffer, pH 4.5) to the sample solution. Deoxygenate by purging with inert gas (N₂ or Ar) for 5-10 minutes.
- Electrode Preparation: Polish the working electrode (e.g., glassy carbon or gold) with alumina slurry (0.05 μm) and rinse thoroughly. For mercury film electrodes, electrodeposit a thin mercury film by reducing Hg²⁺ at a constant potential.
- Preconcentration/Deposition Step: Apply a negative deposition potential (specific to the target metal) to the working electrode under stirring for a defined time (30-300 seconds). During this step, metal ions are reduced and concentrated into the mercury electrode or film.
- Equilibration: Stop stirring and allow the solution to become quiescent for 10-30 seconds.
- Stripping Step: Apply a positive-going potential sweep (linear sweep or differential pulse) from the deposition potential to a more positive potential. As the potential increases, concentrated metals are oxidized (stripped) back into solution, generating characteristic current peaks.
- Quantification: Measure the peak current for each metal, which is proportional to its concentration in the sample. Construct a calibration curve using standard solutions with known metal concentrations.
For organic pharmaceutical compounds, cyclic voltammetry and differential pulse voltammetry are more commonly employed. These methods typically involve:
- Optimization of Experimental Parameters: Including pH, supporting electrolyte, scan rate, and pulse parameters to maximize sensitivity and selectivity.
- Electrode Modification: With nanomaterials (e.g., carbon nanotubes, graphene) or molecularly imprinted polymers to enhance selectivity toward target drug molecules.
- Validation: Against standard reference methods (e.g., HPLC, LC-MS) to ensure accuracy and reliability.
Diagram 1: Potentiometric Sensor Development Workflow
Advanced Sensing Technologies and Applications
Emerging Platforms for TDM and Electrolyte Monitoring
Wearable Potentiometric Sensors represent a transformative advancement enabling continuous monitoring of biomarkers, electrolytes, and pharmaceuticals in biological fluids [7]. These devices leverage solid-contact ion-selective electrodes integrated into flexible substrates that can be directly attached to skin or incorporated into clothing [7]. Key advantages include non-invasive or minimally invasive sampling, real-time data acquisition, and potential for closed-loop therapeutic systems [7]. Recent innovations in this area include sweat-based sensors for electrolyte monitoring and transdermal systems for drug level tracking [7] [59].
Paper-Based Potentiometric Devices offer cost-effective, versatile platforms for point-of-care analysis, facilitating rapid determination of various analytes in resource-limited settings [7]. These devices typically incorporate microfluidic channels and sensing zones fabricated on paper substrates, with integrated reference and indicator electrodes [7]. Applications include monitoring of critical electrolytes (Na⁺, K⁺, Cl⁻) and TDM for drugs with narrow therapeutic indices [7].
3D Printing Technologies have emerged as powerful tools for potentiometric sensor fabrication, providing enhanced flexibility, precision in manufacturing ion-selective electrodes, and rapid prototyping capabilities that accelerate optimization of electrochemical parameters [7]. Additive manufacturing approaches enable customized sensor geometries, multi-analyte arrays, and integration of complex fluidic pathways for sample handling [7].
Electrochemical Nanosensors represent a cutting-edge development in TDM technologies, offering reliable quantitative analysis at clinically relevant concentrations [58] [57]. These nanobiosensors exhibit transformative potential in healthcare through their enhanced sensitivity, miniaturization capabilities, and compatibility with portable instrumentation [58]. Nanomaterials employed in these sensors include metal nanoparticles, quantum dots, carbon nanotubes, and graphene, which improve electron transfer kinetics and allow for surface functionalization with specific recognition elements [58].
Table 2: Performance Comparison of Advanced Sensing Platforms
| Platform | Key Features | Analytes | Detection Limits | Advantages |
|---|---|---|---|---|
| Wearable Potentiometric Sensors | Continuous monitoring, flexible substrates, solid-contact ISEs | Electrolytes (Na⁺, K⁺, Ca²⁺), pharmaceuticals | ~μM range for ions [7] | Real-time data, non-invasive potential, continuous monitoring |
| Paper-Based Devices | Low-cost, disposable, microfluidic capabilities | Point-of-care electrolytes, TDM for specific drugs | ~mM range for ions [7] | Cost-effective, portable, minimal sample requirements |
| 3D Printed Sensors | Custom geometries, rapid prototyping, multi-analyte capability | Wide range of ionic species and drugs | Comparable to conventional ISEs [7] | Design flexibility, rapid iteration, integration potential |
| Electrochemical Nanosensors | Nanomaterials, enhanced sensitivity, surface functionalization | Antibiotics, antiepileptics, anticancer drugs | ~nM to pM range for drugs [58] | Ultra-sensitive, miniaturization, multiplexing capability |
Applications in Therapeutic Drug Monitoring
TDM combines the quantification of drug concentrations in biological matrices with pharmacological interpretation to guide treatment decisions, representing a valuable tool in precision medicine [57]. Conventional TDM relies on techniques such as liquid chromatography-mass spectrometry (LC-MS) and immunoassays, which require centralized laboratories, specialized equipment, and trained personnel [57]. Electrochemical sensors offer promising alternatives through their potential for point-of-care testing, rapid response, miniaturization, convenient operation, and portability [58] [57].
Drugs commonly monitored using TDM approaches include:
- Antiepileptic drugs (e.g., phenytoin, carbamazepine, valproic acid) where TDM is essential due to narrow therapeutic windows and concentration-dependent efficacy and toxicity [57].
- Antibiotics (e.g., vancomycin, aminoglycosides) particularly for drugs with concentration-dependent antibacterial activity and potential nephrotoxicity [57].
- Anticancer drugs (e.g., methotrexate, 5-fluorouracil) where TDM helps optimize efficacy while minimizing severe side effects [57].
- Immunosuppressants (e.g., cyclosporine, tacrolimus) requiring precise dosing to maintain therapeutic levels while avoiding toxicity [57].
Electrochemical approaches to TDM primarily utilize two methodological frameworks:
- Direct Detection of electroactive drugs through voltammetric techniques that exploit inherent redox properties of the target molecules.
- Indirect Detection using potentiometric sensors incorporating ionophores with selective binding affinity for specific drug molecules, or voltammetric immunosensors and aptasensors that employ biological recognition elements.
Diagram 2: TDM Process with Electrochemical Detection
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagents and Materials for Electrochemical TDM and Electrolyte Sensing
| Category | Specific Examples | Function/Purpose |
|---|---|---|
| Conducting Polymers | PEDOT:PSS, polyaniline, poly(3-octylthiophene) | Solid-contact layer in SC-ISEs; ion-to-electron transduction [7] |
| Carbon Nanomaterials | Multi-walled carbon nanotubes, graphene, MXenes, mesoporous carbon | Solid-contact transducers; enhance capacitance and stability [7] |
| Ionophores | Valinomycin (K⁺), bis-(12-crown-4) (Na⁺), ionophores for drug molecules | Molecular recognition elements in ISEs; provide selectivity [7] |
| Polymer Matrices | Poly(vinyl chloride), silicone rubber, polyurethanes | Membrane matrix for ion-selective electrodes; provide mechanical stability [7] |
| Plasticizers | 2-Nitrophenyl octyl ether, bis(2-ethylhexyl) sebacate | Impart flexibility to polymer membranes; influence dielectric constant [7] |
| Ionic Additives | Lipophilic salts (e.g., KTpClPB, TDMA⁺TFPB⁻) | Create permselectivity; reduce membrane resistance [7] |
| Electrode Materials | Glassy carbon, gold, platinum, screen-printed electrodes | Working electrode substrates; provide electronic conductivity [56] |
| Reference Electrodes | Ag/AgCl, double-junction reference electrodes | Provide stable reference potential; complete electrochemical cell [7] [43] |
| Biological Recognition Elements | Antibodies, aptamers, enzymes (e.g., cytochrome P450) | Selective binding for voltammetric biosensors; enable drug specificity [57] |
| Nanoparticles | Gold nanoparticles, magnetic nanoparticles, quantum dots | Signal amplification; enhance electron transfer; enable surface modification [58] |
Comparative Analysis: Measurement Principles in Biomedical Applications
The fundamental distinction between current measurement in voltammetry and potential measurement in potentiometry dictates their respective applications in TDM and electrolyte monitoring. This comparative analysis examines the technical considerations for each approach:
Potentiometric Methods excel in continuous monitoring applications due to their equilibrium measurement principle, minimal power requirements, and inherent simplicity [7] [43]. These characteristics make them particularly suitable for wearable sensors and implantable devices where long-term stability and low energy consumption are critical [7]. The logarithmic response of potentiometric sensors (according to the Nernst equation) provides wide dynamic range but limits precision at very low concentrations compared to voltammetric methods [43]. Additionally, potentiometric sensors typically exhibit excellent selectivity toward specific ions when properly formulated with selective ionophores, but may suffer from interference in complex matrices [7].
Voltammetric Methods offer superior sensitivity and lower detection limits, particularly for trace analysis of electroactive species [56]. The direct proportionality between current and concentration provides better precision at low concentration levels compared to potentiometry [56]. Voltammetric techniques also provide rich information about reaction kinetics and mechanisms through variations in potential waveforms [56]. However, these methods generally consume more power, may require more complex instrumentation, and can be susceptible to electrode fouling in biological matrices [56].
Table 4: Comparative Analysis of Measurement Principles
| Characteristic | Potentiometry (Potential Measurement) | Voltammetry (Current Measurement) |
|---|---|---|
| Fundamental Signal | Potential (V) at zero current | Current (A) at applied potential |
| Measurement Conditions | Equilibrium or near-equilibrium | Non-equilibrium (controlled potential) |
| Sensitivity | Moderate (Nernstian limit: ~59 mV/decade) | High (nA-pA range achievable) |
| Detection Limits | ~10⁻⁶ to 10⁻¹¹ M for ions [7] | ~10⁻⁸ to 10⁻¹¹ M for metals [56] |
| Selectivity | Governed by ionophore chemistry | Governed by redox potential and electrode modification |
| Power Consumption | Low (minimal current flow) | Moderate to high (current flow required) |
| Fouling Susceptibility | Generally low | Can be significant in complex matrices |
| Miniaturization Potential | Excellent (size-independent response) [7] | Good, but microelectrode effects must be considered |
| Continuous Monitoring | Well-suited for long-term monitoring | Limited by fouling and consumption of analyte |
| Multiplexing Capability | Straightforward with ion-selective arrays | Possible with electrode arrays and potential programming |
Future Perspectives and Research Directions
The convergence of electrochemical sensing technologies with advanced materials science, microfabrication, and data analytics is driving several emerging trends in TDM and electrolyte monitoring:
Multimodal Sensing Systems that integrate multiple sensing principles (e.g., potentiometric, voltammetric, and optical detection) on a single platform offer comprehensive analytical capabilities by leveraging the complementary strengths of each technique [59]. These systems can simultaneously monitor drug concentrations, electrolyte levels, and physiological parameters, providing a more complete picture of patient status [59].
Closed-Loop Therapeutic Systems represent the ultimate application of continuous monitoring technologies, where real-time sensor data automatically adjusts drug delivery rates to maintain therapeutic concentrations [57]. Such systems require extremely reliable sensors with minimal drift, rapid response times, and robust performance in biological environments [57].
Artificial Intelligence-Enhanced Data Interpretation is increasingly important for extracting meaningful information from complex sensor arrays and compensating for non-specific interactions in biological matrices [59]. Machine learning algorithms can identify patterns in multidimensional sensor data, improve quantification accuracy, and potentially predict individual pharmacokinetic parameters [59].
Standardization and Clinical Validation remain critical challenges for translating electrochemical sensors from research laboratories to clinical practice [58] [57]. Future research must address reproducibility, manufacturing scalability, rigorous validation against gold-standard methods, and demonstration of clinical utility through appropriately designed trials [58] [57].
The integration of electrochemical sensing platforms within N-of-1 clinical trial designs offers particularly promising opportunities for personalized medicine [57]. These approaches treat each patient as an independent study to determine optimal treatment regimens based on individual pharmacokinetic and pharmacodynamic responses [57]. The continuous, real-time data provided by advanced electrochemical sensors perfectly aligns with this paradigm, potentially enabling unprecedented personalization of drug therapies.
Electroanalytical techniques, primarily voltammetry and potentiometry, form the cornerstone of modern chemical sensing. Their significance is increasingly pronounced in the development of next-generation wearable sensors and 3D-printed electrochemical devices. These techniques are fundamentally differentiated by their measurement parameters: voltammetry involves applying a time-dependent potential and measuring the resulting current arising from faradaic reactions, while potentiometry measures the equilibrium potential across an ion-selective membrane at near-zero current [7] [60] [61]. This distinction is critical for application-specific sensor design. The advent of flexible electronics and additive manufacturing has catalyzed the convergence of these techniques into platforms for real-time, on-body health monitoring and customized, high-performance energy storage and sensing systems [7] [62] [63]. This whitepaper provides an in-depth technical guide to these emerging applications, framed within the core principles of current and potential measurement, and details the experimental methodologies propelling the field forward.
Theoretical Foundations: Voltammetry vs. Potentiometry
The operational principles of voltammetry and potentiometry dictate their respective niches in sensing applications. Understanding these fundamentals is essential for selecting the appropriate technique for a given analytical challenge, particularly in wearable and 3D-printed contexts.
Voltammetric Methods
Voltammetry is a dynamic technique where a time-dependent potential is applied to a working electrode, and the resulting current from the oxidation or reduction of an analyte is measured [60] [64]. The resulting plot of current versus applied potential is called a voltammogram. The applied potential provides the energy to drive electron transfer, and the measured current is proportional to the concentration of the electroactive species. Key techniques include:
- Linear Sweep Voltammetry (LSV): The potential is swept linearly from a start to an end potential [64].
- Cyclic Voltammetry (CV): The potential is swept linearly to a vertex potential and then swept back, providing information on redox potentials and reaction kinetics [65].
- Differential Pulse Voltammetry (DPV): Small potential pulses are superimposed on a linear baseline, and the current difference is measured to minimize charging current and enhance sensitivity [12].
- Square Wave Voltammetry (SWV): A square waveform is applied, offering very fast scan speeds and excellent sensitivity [12].
- Anodic Stripping Voltammetry (ASV): A pre-concentration step is used where analytes are electroplated onto the electrode, followed by a stripping step that provides very low detection limits for trace metals [12].
The relationship between current, potential, and concentration is governed by equations such as the Butler-Volmer equation (for kinetics) and the Cottrell equation (for diffusion-controlled currents) [64] [12]. Voltammetry typically employs a three-electrode system—working, counter, and reference electrodes—to precisely control the working electrode potential [60] [64].
Potentiometric Methods
In contrast, potentiometry is a static technique that measures the potential difference between two electrodes under conditions of zero or negligible current flow [7] [61]. This potential is related to the analyte activity by the Nernst equation. The core component is the ion-selective electrode (ISE), which generates a membrane potential selective to a particular ion. Potentiometric sensors are classified into:
- Liquid-Contact ISEs (LC-ISE): Feature an internal filling solution contacting the ion-selective membrane [7].
- Solid-Contact ISEs (SC-ISE): The internal solution is replaced by a solid-contact layer that acts as an ion-to-electron transducer, making them more robust and suitable for miniaturization and wearable applications [7].
Solid-contact layers often use materials like conducting polymers (e.g., PEDOT:PSS) or carbon-based nanomaterials (e.g., graphene, carbon nanotubes) to provide high capacitance and stability [7] [66].
Table 1: Fundamental Comparison of Voltammetry and Potentiometry.
| Feature | Voltammetry | Potentiometry |
|---|---|---|
| Measured Signal | Current (i) | Potential (E) |
| Current Flow | Significant (Faradaic) | Negligible (Zero-Current) |
| Primary Equation | Butler-Volmer, Cottrell | Nernst |
| Electrode System | Three-electrode system | Two-electrode system (Working & Reference) |
| Selectivity | Achieved via applied potential and electrode material | Achieved via ion-selective membrane |
| Sensitivity | Very high (e.g., nM-pM for stripping techniques) | Good (e.g., µM-mM) |
| Temporal Resolution | Excellent for real-time dynamics | Suited for continuous monitoring |
Diagram 1: Technique selection workflow for voltammetry and potentiometry.
Emerging Application 1: Wearable Electrochemical Sensors
The drive for personalized, real-time health monitoring has made wearable sensors a premier application for electrochemical techniques. The choice between voltammetric and potentiometric approaches is dictated by the target analyte and the required measurement regime.
Wearable Potentiometric Sensors
Wearable potentiometric sensors are predominantly used for the continuous monitoring of ionic species (e.g., K⁺, Na⁺, Ca²⁺, H⁺, Cl⁻) in biofluids like sweat, tears, or interstitial fluid [7] [62]. Their low power consumption and simplicity make them ideal for long-term, on-body sensing.
- Design Principle: Typically implemented as solid-contact ion-selective electrodes (SC-ISEs) to eliminate the internal filling solution, which is mechanically unstable for wearables [7]. The critical components are:
- Substrate: A flexible material (e.g., polyester, polyimide, textile).
- Solid-Contact Layer: Transduces ionic signal to electronic signal; common materials include conducting polymers (PEDOT:PSS) and carbon nanomaterials.
- Ion-Selective Membrane (ISM): Contains an ionophore for selective recognition of the target ion.
- Applications: Monitoring electrolytes in sweat for hydration status, assessing sodium/potassium levels for neuromuscular and metabolic function, and point-of-care therapeutic drug monitoring (TDM) of pharmaceuticals with a narrow therapeutic index [7].
- Experimental Protocol: Fabrication of a Textile-Based Sweat Potassium Sensor.
- Electrode Preparation: A flexible polyester sheet with pre-patterned carbon ink electrodes serves as the platform.
- Solid-Contact Deposition: The working electrode area is drop-cast with a dispersion of PEDOT:PSS and allowed to dry, forming the transducer layer.
- Membrane Casting: An ion-selective membrane cocktail is prepared by dissolving high-molecular-weight PVC, a plasticizer (e.g., DOS), and a valinomycin (K⁺ ionophore) in tetrahydrofuran (THF). This cocktail is drop-cast onto the PEDOT:PSS layer and left to evaporate overnight to form a stable ISM.
- Integration: The sensor is integrated into a sweat band along with a Ag/AgCl reference electrode.
- Calibration & Measurement: The sensor is calibrated in standard KCl solutions (e.g., 0.1 mM to 100 mM). During use, the potential difference between the ISE and the reference electrode is logged continuously by a potentiometer circuit, and the potassium concentration is calculated using the Nernst equation [7] [62].
Wearable Voltammetric Sensors
Wearable voltammetric sensors are employed for detecting electroactive metabolites and biomarkers, such as glucose, lactate, uric acid, and neurotransmitters, often with higher sensitivity and the ability for multi-analyte detection [62] [12].
- Design Principle: Utilize a three-electrode system (working, counter, reference) miniaturized and printed onto a flexible substrate. The working electrode is often functionalized with specific enzymes (e.g., glucose oxidase for glucose) or electrocatalysts.
- Applications:
- Amperometric Sensing: A constant potential is applied, and the steady-state current is measured. This is widely used in continuous glucose monitors (CGMs), where glucose oxidase catalyzes the oxidation of glucose, producing H₂O₂, which is then oxidized at the working electrode [62].
- Pulse Voltammetric Sensing: Techniques like DPV or SWV are used to resolve multiple analytes or reduce fouling effects. For example, DPV can be used for the sensitive detection of uric acid and tyrosine in sweat [62] [12].
- Experimental Protocol: Lactate Sensing in Sweat using Amperometry.
- Electrode Fabrication: Carbon-based working and counter electrodes and a Ag/AgCl reference electrode are screen-printed onto a flexible polyurethane substrate.
- Enzyme Immobilization: The working electrode is modified with a chitosan film containing lactate oxidase (LOx) and a redox mediator (e.g., Prussian Blue) to shuttle electrons.
- Electrochemical Cell Integration: The sensor is housed in a microfluidic sweat collection chamber attached to the skin.
- Measurement: A constant potential of +0.2 V (vs. the integrated Ag/AgCl reference) is applied. The current generated from the enzymatic oxidation of lactate is measured in real-time. The current is proportional to the lactate concentration in the captured sweat [62].
Table 2: Comparison of Wearable Potentiometric and Voltammetric Sensors.
| Parameter | Wearable Potentiometric Sensor | Wearable Voltammetric Sensor |
|---|---|---|
| Primary Signal | Potential | Current |
| Typical Analytes | Ions (Na⁺, K⁺, H⁺, Cl⁻) | Metabolites (Glucose, Lactate), Drugs |
| Selectivity Source | Ionophore in membrane | Enzyme, Applied potential, Mediator |
| Power Consumption | Very Low | Low to Moderate |
| Data Output | Continuous concentration reading | Continuous or pulsed current reading |
| Key Challenge | Signal drift, biocompatibility | Biofouling, enzyme stability |
Emerging Application 2: 3D-Printed Electrochemical Devices
Additive manufacturing, or 3D printing, has introduced a paradigm shift in the design and fabrication of electrochemical devices, enabling the creation of complex, customized 3D architectures that are impossible to achieve with traditional methods like blade-coating [7] [63].
3D Printing in Potentiometry
The primary application of 3D printing in potentiometry is the rapid prototyping and fabrication of customized ion-selective electrodes and all-in-one sensor packages [7].
- Materials and Techniques: Direct Ink Writing (DIW), an extrusion-based method, is the most prominent technique. It allows for the printing of high-viscosity pastes and composites [7] [63].
- Ink Formulation: Carbon-based inks (e.g., graphene, carbon nanotubes) are common, as they can serve as both the conductive electrode and the solid-contact transducer. These inks require specific rheological properties (shear-thinning behavior and high yield stress) to maintain shape after printing [63].
- Device Architecture: 3D printing enables the creation of planar, interdigitated, or bespoke electrode geometries. It also allows for the monolithic printing of the entire electrochemical cell, including the electrode and the sample chamber, onto a substrate [7].
- Experimental Protocol: 3D-Printed All-Solid-State Potassium ISE.
- Ink Preparation: A carbon nanotube (CNT) ink is formulated by dispersing CNTs in a solvent with a cellulose-based binder to achieve a viscoelastic, shear-thinning rheology suitable for DIW.
- Printing Electrodes: A three-axis motion stage with a micro-extrusion printhead is used to deposit the CNT ink onto a substrate, forming the working and counter electrodes layer-by-layer.
- Post-processing: The printed structure is cured at an elevated temperature (e.g., 80°C) to evaporate solvents and solidify the electrode.
- Membrane Functionalization: A potassium-selective membrane cocktail (PVC, plasticizer, valinomycin) is drop-cast onto the printed CNT working electrode.
- Performance Validation: The printed ISE is characterized by measuring its potential response in a series of KCl solutions to determine its slope, linear range, and detection limit [7] [63].
3D Printing in Voltammetry
3D printing is revolutionizing voltammetric devices by fabricating electrodes with high surface area, complex porous networks, and tailored geometries that enhance mass transport and electron transfer [63] [66].
- Architectural Advantages: 3D-printed electrodes can be designed with biomimetic structures (e.g., hierarchical pores, vertically aligned channels) that drastically reduce ion diffusion pathways and increase the electroactive surface area, leading to higher sensitivity and power density in sensing and energy storage applications [63].
- Multi-material Printing: A key advantage is the ability to integrate different functional materials in a single print job. For instance, a working electrode of carbon-black/PLA composite can be printed alongside an insulating polymer body and a Ag/AgCl reference electrode, creating a fully integrated, custom-shaped electrochemical sensor [63].
- Experimental Protocol: Fabrication of a 3D-Printed Microelectrode for Dopamine Sensing.
- Model Design: A microelectrode with a 50 µm diameter disk geometry is designed using computer-aided design (CAD) software.
- Graphene-based Ink Synthesis: A printable graphene oxide (GO) ink is synthesized and loaded into a DIW printer.
- Printing: The microelectrode structure is printed onto a silicon wafer substrate.
- Post-Processing Thermal Reduction: The printed GO structure is converted to conductive reduced graphene oxide (rGO) via a thermal or chemical reduction process.
- Electrochemical Testing: The 3D-printed rGO microelectrode is characterized using cyclic voltammetry (CV) and differential pulse voltammetry (DPV) in a dopamine solution to assess its sensitivity, selectivity against ascorbic acid, and limit of detection, leveraging the enhanced surface area and electrocatalytic properties of the 3D rGO structure [63] [12].
The Scientist's Toolkit: Essential Research Reagents and Materials
The development of advanced wearable and 3D-printed electrochemical devices relies on a specialized set of materials and reagents.
Table 3: Key Research Reagent Solutions for Advanced Electrochemical Devices.
| Material/Reagent | Function | Example Applications |
|---|---|---|
| PEDOT:PSS | Conducting polymer; used as a solid-contact ion-to-electron transducer in potentiometric sensors. | Wearable SC-ISEs for K⁺, Na⁺ [7] [66]. |
| Valinomycin | Potassium ionophore; confers high selectivity for K⁺ over other cations in the ISM. | Potentiometric sweat sensors [7]. |
| Graphene Oxide (GO) / Carbon Nanotube (CNT) Inks | Nanocarbon materials providing conductivity and mechanical strength; base for 3D printing inks. | 3D-printed voltammetric electrodes, supercapacitors [63] [66]. |
| Glucose Oxidase (GOx) / Lactate Oxidase (LOx) | Enzymes that catalyze the oxidation of specific biomarkers (glucose, lactate). | Amperometric biosensors in wearables [62]. |
| Prussian Blue | Electrocatalyst and redox mediator; efficiently reduces the overpotential for H₂O₂ oxidation. | Second-generation amperometric glucose sensors [62]. |
| Polyvinyl Chloride (PVC) & Plasticizers | Polymer matrix and solvent for ion-selective membranes in potentiometry. | Membrane for all solid-contact ISEs [7]. |
| Ionic Liquids | Electrolytes with high stability and wide electrochemical windows; used in gel electrolytes. | Flexible supercapacitors, conductive hydrogels [66]. |
| Poly(vinylidene fluoride) (PVDF) | Piezoelectric polymer; enables self-powered mechanical sensing. | Wearable piezoelectric pressure sensors [62]. |
Diagram 2: Material stacks for wearable and 3D-printed electrochemical devices.
The fields of wearable sensing and 3D-printed electrochemical devices are being profoundly shaped by the fundamental principles of voltammetry and potentiometry. The choice between measuring current and measuring potential dictates the sensor's design, capabilities, and ultimate application. Wearable potentiometry excels in the low-power, continuous monitoring of key electrolytes, while wearable voltammetry provides superior sensitivity for dynamic metabolic monitoring. Concurrently, 3D printing technology is breaking the constraints of traditional manufacturing, allowing for the creation of devices with optimized architectures that enhance both voltammetric and potentiometric performance. The convergence of these fields—guided by a deep understanding of electroanalytical principles and enabled by novel materials—is paving the way for a new generation of personalized health monitoring systems and highly customized analytical devices. Future progress will hinge on multidisciplinary efforts to improve the stability, selectivity, and seamless integration of these sophisticated platforms.
Enhancing Performance: Troubleshooting Sensor Stability and Selectivity
Mitigating Electrode Fouling in Complex Biological Matrices
Electrode fouling presents a fundamental challenge in electrochemical analysis, particularly in complex biological matrices such as blood, serum, saliva, and urine. This phenomenon involves the passivation of electrode surfaces by fouling agents that form impermeable layers, inhibiting direct contact between analytes and the electrode surface and consequently degrading sensor performance [67]. The persistence of fouling manifests as diminished sensitivity, elevated detection limits, poor reproducibility, and unreliable analytical readings [68] [67]. Within the specific context of electrochemical research methodologies, the impact and manifestation of fouling differ significantly between dynamic techniques like voltammetry, which measures current under applied potential, and equilibrium techniques like potentiometry, which measures potential at zero current [1]. This technical guide examines the mechanisms underlying electrode fouling and explores advanced mitigation strategies tailored for these distinct electrochemical approaches, with particular emphasis on applications in pharmaceutical research and drug development.
Table 1: Core Electrochemical Techniques: Operational Principles and Fouling Vulnerability
| Technique | Operating Principle | Primary Measurement | Key Applications | Fouling Vulnerability |
|---|---|---|---|---|
| Voltammetry | Applies a changing potential to the working electrode [1] | Current resulting from redox reactions [1] | Trace metal analysis, drug quantification, reaction mechanism studies [69] [1] | High (due to reaction products and adsorption) [67] |
| Amperometry | Applies a constant potential to the working electrode [1] | Current over time [69] [1] | Glucose biosensors, real-time detection [69] [1] | High (continuous polarization promotes adsorption) |
| Potentiometry | Measures the potential difference at zero current [7] [1] | Potential (EMF) relative to reference electrode [7] [1] | pH, ion-selective electrodes (Na+, K+, Ca2+), therapeutic drug monitoring [7] [1] | Moderate (surface conditioning affects membrane potential) [70] |
Fouling Mechanisms and Underlying Causes
Fouling mechanisms vary considerably based on the electrochemical technique, sample matrix, and electrode material. In voltammetric systems, fouling often occurs when the analyte itself or its electrochemical reaction products actively adsorb onto or polymerize on the electrode surface [67]. For instance, during the detection of neurotransmitters like dopamine, the oxidation product dopaminechrome can polymerize into a melanin-like film that covalently bonds to the electrode surface, irreversibly fouling it [67]. Similarly, the oxidation of phenolic compounds generates radical intermediates that undergo coupling reactions to form dimers, oligomers, and finally, impermeable polymeric layers [67].
In potentiometric systems utilizing ion-selective electrodes (ISEs), fouling typically involves the accumulation of organic, inorganic, or biological material on the ion-selective membrane [70]. This accumulation physically blocks ion transport pathways and alters the membrane potential, leading to signal drift. In biological fluids, proteins adsorb to surfaces through hydrophobic, hydrophilic, and electrostatic interactions, forming a conditioning film that facilitates further biofouling [67] [70]. The subsequent adhesion of cells, cell fragments, and other biological macromolecules culminates in biofilm formation, which significantly alters the local chemical environment at the electrode interface [71] [70].
Diagram 1: Fouling Mechanisms Pathway in Complex Biological Matrices. This diagram illustrates the sequential processes from sample introduction to performance degradation.
The intrinsic properties of the electrode material significantly influence fouling susceptibility. Hydrophobic electrode surfaces (e.g., diamond, carbon nanotubes) promote fouling by hydrophobic species like aromatic compounds and proteins, which unfold to expose their hydrophobic residues in aqueous environments [67]. This hydrophobic interaction is entropically favorable and often leads to irreversible fouling. In contrast, fouling mediated by hydrophilic or electrostatic interactions tends to be more reversible, as water molecules compete for these binding sites [67].
Comparative Fouling Dynamics: Voltammetry vs. Potentiometry
The fundamental operational differences between voltammetry and potentiometry dictate their respective vulnerabilities and responses to fouling. Voltammetry, being a dynamic technique that involves electron transfer reactions, is highly susceptible to fouling from the analyte and its reaction products [67] [1]. The applied potential can drive the formation of insoluble polymeric films that directly passivate the active electrode surface. This fouling layer increases the electron transfer resistance, leading to a diminished faradaic current, peak broadening, and a negative shift in peak potential.
Potentiometry, operating at zero current, measures the equilibrium potential across an ion-selective membrane [7] [1]. Fouling in ISEs does not typically block an electron transfer reaction but rather alters the ion-exchange processes at the membrane surface or physically blocks ion transport [70]. This results in a gradual drift in the baseline potential and a decrease in the slope of the sensor's response. While generally less immediately catastrophic than in voltammetry, potentiometric fouling can be equally detrimental for long-term monitoring applications, as it undermines the stability and accuracy required for precise ion activity measurement [70].
Table 2: Fouling Manifestations and Analytical Consequences in Voltammetry vs. Potentiometry
| Aspect | Voltammetric Sensors | Potentiometric Sensors (ISEs) |
|---|---|---|
| Primary Fouling Effect | Increased electron transfer resistance; passivation of active sites [67] | Alteration of ion-exchange kinetics and membrane surface potential [70] |
| Impact on Signal | Decreased faradaic current; shifted peak potentials [67] | Gradual potential drift; reduced slope [70] |
| Key Fouling Agents | Polymerizing analytes (e.g., phenols, dopamine), proteins [67] | Proteins, lipids, biofilms, salt precipitates [70] |
| Typical Onset | Can be rapid (seconds to minutes) [67] | Often gradual (hours to days) [70] |
| Common Mitigation | Pulsed waveforms (DPV, SWV), surface modification, polymer coatings (Nafion) [69] [67] | Hydrophilic/zwitterionic coatings, nanomaterials, photocatalytic layers (TiO₂) [68] [70] |
Experimental Protocols for Fouling Mitigation and Characterization
Protocol: Fabrication of an Antifouling Coating for Voltammetric Electrodes
This protocol details the modification of a glassy carbon electrode (GCE) with a Nafion-polyethylene glycol (PEG) composite coating to mitigate protein fouling in biological fluids [67].
- Electrode Pretreatment: Polish the GCE sequentially with 1.0, 0.3, and 0.05 µm alumina slurry on a microcloth pad. Rinse thoroughly with deionized water between each polish and sonicate in ethanol and deionized water for 1 minute each to remove adsorbed alumina particles.
- Electrochemical Activation: Place the cleaned GCE in a standard three-electrode cell with a suitable electrolyte (e.g., 0.1 M H₂SO₄). Perform cyclic voltammetry (CV) between -0.2 V and +1.0 V (vs. Ag/AgCl) at a scan rate of 100 mV/s until a stable voltammogram characteristic of a clean GCE is achieved.
- Coating Solution Preparation: Prepare a solution containing 0.5% (v/v) Nafion and 1.0% (w/v) PEG (MW ~400 Da) in a 7:3 (v/v) water-ethanol mixture. Sonicate the mixture for 15 minutes to ensure complete dissolution and homogeneity.
- Electrode Modification: Pipette 5.0 µL of the prepared Nafion-PEG solution onto the polished surface of the GCE. Allow the solvent to evaporate at room temperature for 30 minutes, forming a uniform, thin polymeric film.
- Validation and Fouling Test: Characterize the modified electrode using CV and electrochemical impedance spectroscopy (EIS) in a 5 mM K₃Fe(CN)₆/K₄Fe(CN)₆ solution. To test antifouling performance, incubate the electrode in a 10 mg/mL bovine serum albumin (BSA) solution in phosphate-buffered saline (PBS) for 30 minutes. Re-measure the CV and EIS and compare the signals before and after incubation to quantify the degree of fouling resistance. A minimal change in peak current or charge transfer resistance indicates effective antifouling properties.
Protocol: Developing a Photocatalytic Self-Cleaning Potentiometric ISE
This methodology outlines the creation of a titanium dioxide (TiO₂)-coated solid-contact ion-selective electrode with photocatalytic antifouling capabilities, suitable for long-term monitoring [70].
- Solid-Contact Substrate Preparation: Start with a planar gold or glassy carbon substrate. Electrodeposit a layer of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) by cycling the potential in a monomer-containing solution to create a stable solid-contact transducer layer [7].
- TiO₂ Nanomaterial Synthesis: Prepare a TiO₂ sol-gel by the acid-catalyzed hydrolysis of titanium isopropoxide in ethanol. Alternatively, use a dispersion of commercial TiO₂ nanoparticles (e.g., Degussa P25).
- Membrane Casting and Modification: Prepare a standard ion-selective membrane cocktail containing the ionophore (e.g., valinomycin for K⁺), lipophilic salt, plasticizer, and PVC polymer. Incorporate 2% (w/w) of the synthesized TiO₂ nanoparticles into the cocktail. Sonication is critical here to ensure uniform dispersion of the nanoparticles and prevent agglomeration.
- Electrode Fabrication: Drop-cast a precisely measured volume (e.g., 100 µL) of the TiO₂-modified membrane cocktail onto the PEDOT solid-contact substrate. Allow the solvent (typically tetrahydrofuran) to evaporate slowly over 24 hours under ambient conditions to form a dense, homogeneous membrane.
- Photocatalytic Self-Cleaning Validation:
- Electrochemical Characterization: Calibrate the TiO₂-modified ISE and an unmodified control ISE in standard solutions to confirm Nernstian response.
- Induced Fouling: Expose both electrodes to an accelerated fouling solution containing humic acids and bacterial culture for a set period.
- Activated Cleaning: Irradiate the fouled TiO₂-modified ISE with UV light (wavelength ~365 nm) for a defined duration (e.g., 30 minutes). The photocatalytic activity of TiO₂ will generate reactive oxygen species that degrade the organic foulants on the membrane surface.
- Performance Recovery: Re-calibrate both electrodes. The TiO₂-modified ISE should demonstrate significant recovery of its original potentiometric response (slope and potential), while the control ISE shows permanent signal drift.
Reagent Solutions for Fouling Mitigation Research
Table 3: Essential Research Reagents for Antifouling Sensor Development
| Reagent / Material | Function in Research | Key Characteristics | Example Application |
|---|---|---|---|
| Nafion | Cation-exchange polymer coating; repels negatively charged proteins and biomolecules [67] | Perfluorosulfonic acid; chemically stable; selective permeability [71] | Voltammetric drug detection in serum [67] |
| Polyethylene Glycol (PEG) | Hydrophilic polymer brush layer; creates a hydration barrier against protein adsorption [67] | High chain mobility; neutral and biocompatible; steric repulsion effect | Antifouling coatings on gold electrodes [68] |
| Titanium Dioxide (TiO₂) | Photocatalytic nanoparticle; generates reactive oxygen species to degrade organic foulants under UV light [70] | Semiconductor; strong oxidative power; self-cleaning effect | Photocatalytic self-cleaning ISE membranes [70] |
| Conducting Polymers (PEDOT) | Solid-contact ion-to-electron transducer in ISEs; can be engineered with antifouling properties [7] [67] | High capacitance; stable potential; compatible with membrane materials | Solid-contact ion-selective electrodes [7] |
| Zwitterionic Materials | Form super-hydrophilic surfaces that strongly bind water, creating a physical and energetic barrier to fouling [68] [70] | Charge-neutral; high hydration capacity; resistant to protein adsorption | Ultra-low fouling coatings for implantable sensors [68] |
Advanced Strategies and Future Perspectives
The frontier of antifouling research integrates material science with smart electronics to create adaptive and regenerative sensor interfaces. Future strategies focus on moving from passive protection to active management of the electrode surface.
- Stimuli-Responsive Surfaces: "Smart" polymer coatings that can change their conformation and properties (e.g., from hydrophilic to hydrophobic) in response to external triggers like temperature, pH, or light offer dynamic fouling control. This allows for on-demand release of adsorbed foulants or switching to a non-fouling state during measurement cycles [68].
- Integrated Fouling Detection and Cleaning: Combining electrochemical sensors with real-time fouling diagnostics, such as non-Faradaic EIS, enables closed-loop systems. The sensor can autonomously trigger a cleaning protocol (e.g., electrochemical potential pulsing or activation of a photocatalytic layer) upon detecting a predefined level of signal degradation, thereby maintaining long-term stability [70].
- Advanced Nanocomposites: The development of multi-functional nanocomposites is a key trend. For example, combining the high capacitance of carbon nanotubes with the hydrophilicity of zwitterionic polymers and the electrocatalytic properties of metal nanoparticles creates synergistic effects, enhancing both sensing performance and fouling resistance [7] [68].
- Bioinspired and Biomimetic Approaches: Learning from nature, researchers are developing coatings with micro- and nano-topographies that mimic shark skin or lotus leaves to prevent biofilm attachment. The use of natural antifoulants like capsaicin or zosteric acid embedded in sensor membranes provides an eco-friendly alternative to toxic biocides [70].
Diagram 2: Antifouling Strategy Evolution from Foundational to Advanced Concepts. This diagram shows the progression from current mitigation methods toward future integrated systems.
The convergence of these advanced strategies is paving the way for robust, maintenance-free electrochemical sensors. For researchers in drug development, this translates to the potential for highly reliable, continuous monitoring of pharmacokinetics, therapeutic drug levels, and critical biomarkers in biologically complex environments, ultimately enhancing drug safety and efficacy studies.
The accurate detection of specific target analytes in complex, multi-component samples is a fundamental challenge in electroanalysis. Interfering ions and molecules, which are often present in biological, environmental, or pharmaceutical samples, can generate non-specific signals, leading to inaccurate quantification and false positives. The core of this challenge lies in designing sensor systems and methodologies that can robustly discriminate the target signal from noise and interference. The approach to achieving this selectivity is intrinsically linked to the fundamental electrochemical technique employed—whether it involves measuring current, as in voltammetry, or measuring potential, as in potentiometry. This guide provides an in-depth examination of the advanced strategies and experimental protocols used to optimize selectivity within the context of this broader methodological framework.
Core Principles: Current vs. Potential Measurement
The choice between voltammetry and potentiometry dictates the fundamental strategy for overcoming interference.
Voltammetry is an active technique where a controlled potential is applied to the working electrode, and the resulting current from redox reactions is measured. Its selectivity is derived from:
- Applied Potential: The potential at which a species is oxidized or reduced serves as a primary fingerprint.
- Kinetic Control: The rate of electron transfer can be tuned to favor the target analyte.
- Mass Transport: How the analyte arrives at the electrode surface (e.g., diffusion, convection) can be exploited [72].
Potentiometry is a passive technique where the potential difference across an electrochemical cell is measured at zero or near-zero current. This potential, governed by the Nernst equation, relates to the activity of ions in solution. Selectivity is achieved almost exclusively through the membrane composition of ion-selective electrodes (ISEs), which is designed to thermodynamically prefer the primary ion over interferents [43] [73].
Table 1: Fundamental Differences in Selectivity Approaches
| Feature | Voltammetry (Current Measurement) | Potentiometry (Potential Measurement) |
|---|---|---|
| Basis of Selectivity | Applied potential window, electrode kinetics, mass transport | Membrane thermodynamics (ionophore selectivity) |
| Key Selectivity Parameter | Peak potential ((E_p)) | Selectivity coefficient ((K_{ij}^{pot})) |
| Role of Interferents | Cause overlapping faradaic currents | Compete for binding sites at the membrane-sample interface |
| Typical Data Output | Voltammogram (Current vs. Potential) | Calibration Curve (Potential vs. log[Activity]) |
Advanced Strategies for Selectivity Optimization
Voltammetric Techniques
1. Waveform Engineering and Multivariate Analysis A powerful method to disambiguate signals involves using a double waveform in Fast-Scan Cyclic Voltammetry (FSCV). One waveform is designed to be insensitive to the target analyte but sensitive to the interference (e.g., pH change), while a second waveform detects both. A Partial Least Squares Regression (PLSR) model is then used to predict and subtract the interfering signal. This approach has been successfully demonstrated for distinguishing hydrogen peroxide (H₂O₂) fluctuations from local pH changes in the brain, a common confounding factor [74].
2. Electrode Modification and Material Science Modifying the electrode surface with nanomaterials or polymers is a primary route to enhanced selectivity. These materials can pre-concentrate the target analyte, catalyze its reaction, or block interferents.
- Nanocomposite Materials: A flower-like WS₂-WO₃/Poly-2-aminobenzene-1-thiol (P2ABT) nanocomposite was used for Hg²⁺ detection. The negative charge on the nanocomposite physically attracted Hg²⁺ ions, while showing no significant response to interfering ions like Zn²⁺, Ni²⁺, Ca²⁺, Mg²⁺, Al³⁺, and K⁺ in cyclic voltammetry tests [75].
- Conducting Polymers: A glassy carbon electrode modified with 2-amino nicotinamide (2-AN) was developed for the sensitive detection of 2-nitrophenol (2-NP). The modifier's functional groups (–NH₂, –CONH₂) enable strong interactions with 2-NP via hydrogen bonding and π–π interactions, enriching the analyte at the electrode surface and enhancing the electron transfer, thereby improving sensitivity and selectivity [76].
3. Optimization of Operational Parameters Systematically optimizing voltammetric parameters like pulse amplitude, frequency, and step potential is crucial. The Response Surface Methodology (RSM) is an efficient statistical technique that minimizes the number of experiments needed to find the optimal parameter set that maximizes the signal-to-interference ratio. This has been applied in the square-wave voltammetric (SWV) determination of 2-NP and the detection of Isoniazid (INH) using a PEDOT-modified gold electrode [76] [77].
Potentiometric Techniques
1. Backside Calibration Potentiometry This novel method addresses the drift and recalibration challenges of traditional direct potentiometry in complex samples. It uses a thin supported liquid membrane and exploits the buildup of steady-state concentration profiles. The sample ion activity is determined by varying the composition of the inner reference solution until the potential drift upon stirring disappears, indicating a symmetric system with no net ion flux across the membrane. This method has been validated for determining Pb²⁺ in environmental samples where H⁺ is the dominant interferent [73].
2. Ion-Selective Membrane Engineering The selectivity of an ISE is dictated by the ionophore within the polymeric membrane. Research focuses on synthesizing novel ionophores with highly specific molecular recognition sites for the primary ion. The composition of the membrane—including the polymer (e.g., PVC), plasticizer, and lipophilic additives—is also fine-tuned to optimize the selectivity coefficient ((K_{ij}^{pot})), which quantitatively defines the electrode's preference for the primary ion (I) over an interfering ion (J) [43] [73].
Detailed Experimental Protocols
This protocol is for researchers needing to deconvolute overlapping electrochemical signals in real-time.
Workflow Overview:
1. Electrode and System Setup:
- Working Electrode: Fabricate a cylindrical carbon-fiber microelectrode.
- Reference Electrode: Ag/AgCl pellet.
- Counter Electrode: Platinum wire.
- Setup: Use a flow-injection analysis system housed in a Faraday cage. Position the working electrode in a flow cell with a continuous buffer stream (e.g., TRIS buffered saline, pH 7.4) at 1 mL/min.
2. Data Acquisition with Double Waveform:
- Program a potentiostat to apply a custom double triangular waveform at 10 Hz:
- Small Waveform (sWF): Scan from -0.4 V to +0.8 V (H₂O₂ is electrochemically silent in this window).
- Large Waveform (lWF): Scan from -0.4 V to +1.4 V (both H₂O₂ and ΔpH are active).
- Use a high scan rate (e.g., 400 V/s). The two scans are separated by a brief hold (e.g., 12 ms) at the initial potential.
- Collect training data by making 2-second bolus injections of known concentrations of H₂O₂ and solutions at different pH levels.
3. PLSR Model Building and Validation:
- Use the current data from the sWF as the predictor variable (X) to model the ΔpH component.
- Use the current data from the lWF as the response variable (Y).
- Construct a PLSR model to relate X and Y. The model learns to predict the ΔpH contribution in the lWF data based on the sWF data.
- Validate the model's predictive power using k-fold cross-validation (e.g., 5-fold).
4. Signal Deconvolution:
- For an unknown sample, collect voltammetric data using the double waveform.
- Input the sWF data into the trained PLSR model to predict the ΔpH signal in the lWF data.
- Subtract the predicted ΔpH signal from the total lWF signal. The residual signal is the quantified H₂O₂ concentration dynamics.
This protocol outlines the creation and use of a modified electrode for selective heavy metal detection.
Workflow Overview:
1. Synthesis of WS₂-WO₃/P2ABT Nanocomposite:
- Dissolve 0.06 M of the monomer 2-aminobenzene-1-thiol (2ABT) in 1.0 M HCl.
- Add an oxidizing agent mixture of 0.06 M Na₂WO₄ and 0.14 M K₂S₂O₈.
- Allow the oxidative polymerization reaction to proceed at ambient temperature for 24 hours. This integrates WO₃ and WS₂ into the polymer matrix, forming the final nanocomposite thin film.
2. Sensor Fabrication and Characterization:
- Use the synthesized nanocomposite directly as the sensing element in a two-electrode cell (for potentiometry) or as the working electrode in a three-electrode cell (for voltammetry).
- Characterize the modified surface using techniques like Scanning Electron Microscopy (SEM) and Fourier-Transform Infrared Spectroscopy (FTIR) to confirm successful modification.
3. Potentiometric Sensing (Two-Electrode Cell):
- Setup: Use the WS₂-WO₃/P2ABT nanocomposite as the working electrode and a calomel electrode (Hg/Hg₂Cl₂) as the reference.
- Measurement: Immerse the electrode pair in Hg²⁺ solutions with concentrations ranging from 10⁻⁶ M to 10⁻¹ M. Measure the potential at zero current.
- Calibration: Plot the measured potential vs. the logarithm of the Hg²⁺ concentration. A linear Nernstian response (slope of ~29.6 mV/decade for n=2 at 25°C) is expected. The reported slope for this sensor was 33.0 mV/decade [75].
4. Voltammetric Validation and Selectivity Test (Three-Electrode Cell):
- Setup: Use the nanocomposite as the working electrode, a calomel reference electrode, and a graphite counter electrode.
- Measurement: Perform Cyclic Voltammetry (CV) with Hg²⁺ concentrations from 10⁻⁶ M to 10⁻¹ M. Observe the increase in the cathodic peak current, typically around 0.1 V.
- Interference Study: Add high concentrations of potential interfering ions (e.g., Zn²⁺, Ni²⁺, Ca²⁺, Mg²⁺, Al³⁺, K⁺) to the Hg²⁺ solution and record the CV response. A selective sensor will show no significant change in the Hg²⁺ signal.
The Scientist's Toolkit: Essential Research Reagents & Materials
Table 2: Key Reagents and Materials for Electrochemical Selectivity Studies
| Reagent/Material | Function/Application | Example Use Case |
|---|---|---|
| Carbon-Fiber Microelectrode | Working electrode for high temporal/spatial resolution in-vivo sensing. | Distinguishing H₂O₂ from pH changes in the brain [74]. |
| Ion-Selective Membrane Components (Ionophore, PVC, Plasticizer) | Forms the core sensing element of a potentiometric sensor, determining selectivity. | Backside calibration potentiometry for Pb²⁺ detection [73]. |
| 2-Aminobenzene-1-thiol (2ABT) | Monomer for synthesizing a conductive polymer nanocomposite. | Fabrication of WS₂-WO₃/P2ABT sensor for Hg²⁺ [75]. |
| Sodium Tungstate (Na₂WO₄) | Precursor for tungsten oxide (WO₃) and source of tungsten for tungsten disulfide (WS₂). | Synthesis of WS₂-WO₃/P2ABT nanocomposite [75]. |
| 2-Amino Nicotinamide (2-AN) | Modifier for glassy carbon electrodes; provides interaction sites for analytes. | Sensitive determination of 2-nitrophenol [76]. |
| EDOT Monomer (3,4-Ethylenedioxythiophene) | Monomer for electropolymerization to form PEDOT conducting polymer films. | Creating PEDOT-modified gold electrodes for drug (e.g., Isoniazid) detection [77]. |
| Partial Least Squares Regression (PLSR) | Multivariate statistical model for deconvoluting overlapping signals. | Predicting and subtracting pH interference from H₂O₂ voltammetric data [74]. |
Performance Comparison and Data Presentation
The effectiveness of these optimization strategies is quantified through key analytical figures of merit.
Table 3: Performance Metrics of Selectivity-Enhanced Electrochemical Sensors
| Target Analyte | Sensor/S Technique | Key Selectivity Feature | Linear Range | Limit of Detection (LOD) | Reported Selectivity Data |
|---|---|---|---|---|---|
| Hg²⁺ | WS₂-WO₃/P2ABT Nanocomposite [75] | Negative surface charge & material specificity | Potentiometry: 10⁻⁶ to 10⁻¹ M | Not Specified | No significant effects from Zn²⁺, Ni²⁺, Ca²⁺, Mg²⁺, Al³⁺, K⁺ |
| 2-Nitrophenol (2-NP) | 2-AN/GC Electrode with SWV [76] | Surface modification & RSM-optimized parameters | 9.9 nM - 52.5 μM & 52.5 μM - 603 μM | 2.92 nM | Successful detection in tap & river water (Recovery: 97.1-103.6%) |
| H₂O₂ (in vivo) | Carbon-Fiber Microelectrode with DW-PLSR [74] | Double Waveform & Multivariate Analysis | Demonstrated in-vivo | High temporal resolution | Effectively discriminated from concurrent ΔpH signals in brain tissue |
| Isoniazid (INH) | PEDOT/Au Electrode [77] | Catalytic properties of PEDOT polymer | 0.05 - 2 μM | Not Specified | Peak current ~4x higher than bare Au, improved specificity in pharmaceuticals |
Improving Sensor Stability and Lifespan in Solid-Contact Ion-Selective Electrodes (SC-ISEs)
Solid-contact ion-selective electrodes (SC-ISEs) represent a significant advancement over traditional liquid-contact ion-selective electrodes by eliminating the internal filling solution, which enables easier miniaturization, portability, and enhanced stability for field deployments [78]. Despite these advantages, SC-ISEs face persistent challenges that can compromise their stability and operational lifespan, including potential drift, water layer formation, and signal instability [78] [79]. These issues become particularly critical when comparing the fundamental operational principles of potentiometry and voltammetry. Potentiometric sensors measure the potential difference at zero current, following the Nernst equation, where stability is paramount for maintaining a consistent potential reading over time [7] [1]. In contrast, voltammetric techniques measure current as a function of applied potential, which can be less susceptible to certain instability mechanisms but requires different sensor design considerations [48].
The water layer formation at the interface between the ion-selective membrane (ISM) and the solid-contact (SC) layer is a primary culprit behind potential drift. This layer creates an undefined electrolyte environment that destabilizes the electrode potential [78] [79]. Furthermore, insufficient ion-to-electron transduction efficiency and poor interfacial adhesion can lead to high impedance and signal instability, ultimately reducing the sensor's lifespan [7] [79]. Addressing these challenges requires a multi-faceted approach focusing on material science and interfacial engineering.
Core Strategies for Enhancing SC-ISE Stability
Material Selection for the Solid-Contact Layer
The solid-contact layer is crucial for efficient ion-to-electron transduction and preventing water infiltration. Recent research has identified several promising material classes, each with distinct advantages, as summarized in Table 1.
Table 1: Performance Comparison of Solid-Contact Materials for SC-ISEs
| Material Class | Example Materials | Key Advantages | Reported Performance | Lifespan Evidence |
|---|---|---|---|---|
| Conducting Polymers | Polypyrrole [80], PEDOT [81], Poly(3-octylthiophene) [80] | High redox capacitance, reversible doping/dedoping, stable potential | Low potential drift (e.g., stable over 3 months for nitrate sensor [80]) | Retained function after dry storage; multiple calibration cycles [80] [81] |
| Carbon Nanomaterials | Laser-Induced Graphene (LIG) [79], Carbon nanotubes [7] [79], Mesoporous carbon [7] | High double-layer capacitance, chemical inertness, large surface area | High capacitance, enhanced signal stability | Excellent long-term stability with minimal drift [79] |
| Nanocomposites | LIG@TiO2-MXene [79], Fe3O4-MoS2 [7], Au-TTF [7] | Synergistic effects, combined high capacitance and hydrophobicity | Ultralow potential drift (< 0.04 mV/h [79]), high stability in sweat | Superior stability during prolonged exposure to complex matrices [79] |
Interfacial Engineering and Membrane Optimization
Beyond the solid-contact layer, the composition and properties of the ion-selective membrane and its interface with the SC layer are critical.
Enhancing Hydrophobicity: A primary strategy to suppress the detrimental water layer is to engineer highly hydrophobic interfaces. For instance, incorporating block copolymers like SEBS into traditional PVC membranes significantly improves hydrophobicity and mechanical strength, reducing water uptake and potential drift to below 0.04 mV/h [79]. Similarly, using PVDF-based electrospun mats transformed into laser-induced graphene creates a composite with intrinsic water-repellent properties [79].
Membrane Composition and Conditioning: The standard ISM consists of a polymer matrix (e.g., PVC), plasticizer, ionophore, and ion exchanger. Optimizing the ratios of these components is vital for selectivity and preventing component leaching [78]. Furthermore, proper conditioning protocols (soaking in a target ion solution) are essential for establishing a stable initial potential. Studies show that even after prolonged dry storage, a sufficient conditioning period can fully restore sensor performance [80].
Experimental Protocols for Stability Assessment
Fabrication of a Stable Polypyrrole-Based Nitrate SC-ISE
This protocol is adapted from a study demonstrating superior stability over three months [80].
- Substrate Preparation: Begin with a screen-printed graphite electrode.
- Electropolymerization of Polypyrrole (PPy) SC Layer:
- Prepare an aqueous solution containing 0.1 M pyrrole monomer and 0.1 M sodium nitrate.
- Use a standard three-electrode system (screen-printed electrode as working electrode, Ag/AgCl reference, Pt counter).
- Apply a constant current density (e.g., 0.5 mA/cm²) for a set duration (e.g., 100 seconds) to deposit a PPy film on the working electrode.
- Rinse the electrode thoroughly with deionized water and air-dry.
- Ion-Selective Membrane (ISM) Coating:
- Prepare an ISM cocktail by dissolving 1–2 wt% ionophore (e.g., TDMA for nitrate), 0.5–1 wt% ionic additive (e.g., NaTFPB), 65 wt% plasticizer (e.g., DOS), and 33 wt% polymer matrix (e.g., PVC) in a volatile solvent like tetrahydrofuran (THF).
- Drop-cast a precise volume (e.g., 50 µL) of the cocktail onto the PPy-modified electrode.
- Allow the solvent to evaporate slowly, covered for 12–24 hours, to form a homogeneous membrane.
- Conditioning: Before first use and after prolonged storage, condition the sensor by soaking in a 0.01 M NaNO₃ solution for at least 4 hours [80].
Chronopotentiometry for Water Layer Testing
A key test to diagnose the formation of a water layer is chronopotentiometry [78].
- Place the fabricated SC-ISE in a reference solution (e.g., 0.01 M target ion).
- Using a potentiostat, apply a small constant current (e.g., ±1 nA) for a set period (e.g., 10–60 seconds).
- Record the potential transient.
- The potential drift (slope of the potential vs. time curve) is calculated. A lower absolute drift value indicates better stability and the absence of a significant water layer. For example, sensors with optimized hydrophobic contacts demonstrate drifts as low as 0.04 mV/h [79].
Long-Term Stability and Calibration
- Procedure: Regularly calibrate the SC-ISE in standard solutions over its intended lifespan (e.g., days to months). Perform calibrations at consistent intervals (e.g., daily or weekly) [80] [81].
- Data Analysis: Plot the calibration curves (potential vs. log of activity) for each cycle. A stable sensor will show minimal parallel shifts and consistent slopes (sensitivity) over time. Overlapping calibration curves across multiple measurement cycles are a hallmark of excellent stability and enable calibration-free operation after the initial characterization [81].
The Scientist's Toolkit: Essential Reagents and Materials
Table 2: Key Research Reagents and Materials for SC-ISE Development
| Item Name | Function / Role | Specific Examples & Notes |
|---|---|---|
| Conducting Polymers | Serves as redox-capacitive solid-contact layer for ion-to-electron transduction [78]. | Polypyrrole (PPy): Electropolymerized from pyrrole monomer [80]. PEDOT:PSS: Commercially available dispersion, often modified [81]. |
| Carbon Nanomaterials | Provides high double-layer capacitance as a solid-contact material; increases surface area and conductivity [7] [79]. | Laser-Induced Graphene (LIG): Patterned via laser on polymer substrates for flexibility [79]. Multi-Walled Carbon Nanotubes (MWCNTs): Used in composites to enhance performance [7] [79]. |
| Ionophores | Key sensing component; selectively binds target ion in the membrane [78] [82]. | TDMA: Common for nitrate selectivity [80]. Valinomycin: Gold standard for potassium selectivity [7]. |
| Polymer Matrices | Forms the backbone of the ion-selective membrane, hosting active components [78]. | Polyvinyl Chloride (PVC): Most common matrix [78] [83]. Polyurethane (PU): Alternative for better adhesion and reduced water uptake [48]. SEBS Copolymer: Blended with PVC to enhance hydrophobicity and mechanical strength [79]. |
| Plasticizers | Provides fluidity to the membrane, facilitating ion mobility and determining membrane dielectric constant [78]. | bis(2-ethylhexyl) sebacate (DOS): Common for its low polarity [78] [83]. Dioctyl phthalate (DOP): Widely used alternative [78] [83]. 2-Nitrophenyl octyl ether (NOPE): Used for higher polarity membranes [78]. |
| Ionic Additives / Exchangers | Imparts permselectivity and reduces membrane resistance [78]. | Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (NaTFPB): Common cation exchanger [78]. Potassium tetrakis(4-chlorophenyl)borate (KTPCIPB): Common cation exchanger [78]. Sodium tetraphenylborate (Na-TPB): Used to form ion-pair complexes in drug-sensing ISEs [83]. |
The stability and lifespan of SC-ISEs are paramount for their reliable application in clinical diagnostics, environmental monitoring, and wearable devices. The transition from traditional potentiometric measurements to more advanced operational modes, including voltammetry, places additional demands on sensor design and interfacial stability [48]. The strategies outlined herein—employing advanced materials like conducting polymers and nanocomposites for the solid contact, engineering hydrophobic interfaces, and optimizing membrane composition—provide a robust roadmap for developing next-generation SC-ISEs.
Future research directions will likely focus on the scalable fabrication of these advanced materials, such as through laser engraving and screen-printing, to ensure reproducibility and cost-effectiveness [79] [81]. Furthermore, the development of calibration-free sensors [81] and their integration into compact, wearable platforms [7] [79] will be crucial for translating laboratory innovations into practical, real-world analytical tools. By systematically addressing the fundamental challenges of potential drift and water layer formation, researchers can significantly enhance the performance and reliability of SC-ISEs across diverse application fields.
The Impact of Scan Rate in Voltammetry and pH in Potentiometry
Electroanalytical techniques are fundamental tools in modern chemical analysis, with voltammetry and potentiometry representing two cornerstone methodologies. These techniques are distinguished by their core measurement principles: voltammetry measures current resulting from electron transfer under a controlled potential, while potentiometry measures potential at equilibrium under zero-current conditions. The scan rate, a critical operational parameter in voltammetry, directly influences current response, mass transport, and electron transfer kinetics. In potentiometry, the pH of a solution fundamentally determines the equilibrium potential measured by ion-selective electrodes according to the Nernst equation. This technical guide examines the instrumental role of these parameters within the broader context of current measurement in voltammetry versus potential measurement in potentiometry research, providing researchers and drug development professionals with advanced experimental frameworks and analytical perspectives.
Theoretical Foundations: Current vs. Potential Measurement
Voltammetry: Dynamic Current Measurement
Voltammetry is a dynamic electrochemical technique that measures current as a function of applied potential. The controlled potential excitation signal induces redox reactions at the working electrode surface, generating a faradaic current that serves as the analytical signal. The three-electrode system—comprising working, reference, and counter electrodes—enables precise potential control while measuring the resulting current, which is quantitatively related to analyte concentration through Faraday's laws. [1] Unlike equilibrium techniques, voltammetric measurements intentionally drive electron transfer processes, making the temporal domain—particularly potential scan rate—a critical experimental variable.
Potentiometry: Equilibrium Potential Measurement
Potentiometry operates on fundamentally different principles, measuring the potential difference between two electrodes under conditions of zero current flow. This potential develops across selective membranes and correlates with the activity of target ions in solution according to the Nernst equation. The most prevalent application is pH measurement using glass electrodes, where the potential difference responds to hydrogen ion activity. [84] [1] Potentiometric measurements occur at thermodynamic equilibrium, where the measured potential represents a steady-state value without net electrochemical transformation of the analyte.
Table 1: Fundamental Differences Between Voltammetry and Potentiometry
| Characteristic | Voltammetry | Potentiometry |
|---|---|---|
| Measured Signal | Current | Potential |
| Current Flow | Significant | Negligible (zero-current) |
| Equilibrium State | Dynamic, non-equilibrium | Thermodynamic equilibrium |
| Key Governing Equation | Faraday's Law | Nernst Equation |
| Primary Application in Guide | Scan Rate Effects | pH Measurement |
The following diagram illustrates the fundamental operational principles and signal pathways for both techniques:
The Role of Scan Rate in Voltammetric Analysis
Fundamental Principles and Kinetic Regimes
Scan rate (v) in voltammetry determines the timescale of experimentation and governs the relative dominance of diffusion versus kinetic control. In cyclic voltammetry, the relationship between peak current (iₚ) and scan rate reveals fundamental information about the electrochemical process:
- Diffusion-controlled processes exhibit a linear relationship between iₚ and v¹/²
- Surface-confined processes show a linear relationship between iₚ and v
- Heterogeneous electron transfer kinetics are determined by the peak potential separation (ΔEₚ) dependence on scan rate
The scan rate directly controls the diffusion layer thickness, with faster scan rates producing thinner diffusion layers and higher concentration gradients, resulting in increased peak currents. [85]
Advanced Scan Rate Methodologies
Rapid Scan Cyclic Voltammetry (RSCV) coupled with ultramicroelectrodes (UMEs) represents a cutting-edge approach for investigating transient reaction intermediates. This combination enhances mass transport via three-dimensional hemispherical diffusion, reduces capacitive currents, and minimizes iR drop. [85] A recent RSCV study investigating the oxygen reduction reaction (ORR) on gold UMEs in alkaline media demonstrated how varying scan rates from 0.1 to 10 V/s alters reaction pathways, enabling quantification of peroxide anion (HO₂⁻) formation rates. [85] At high scan rates, the system transitions from steady-state to transient diffusion control, capturing short-lived intermediates that would be undetectable at conventional scan rates.
Experimental Protocol: Optimizing Scan Rate for Analyte Detection
Objective: Determine optimal scan rate for sensitive cocaine detection using square wave voltammetry (SWV) on modified screen-printed electrodes. [86]
Materials and Equipment:
- PalmSens 4 Potentiostat with PSTrace software
- Screen-printed carbon electrodes (Zensor R&D)
- Cocaine hydrochloride standard solutions (Sigma-Aldrich)
- Phosphate buffer saline (PBS, pH 7.4)
- Human saliva samples (collected fresh)
Methodology:
- Electrode Modification: Drop-cast 5 μL cocaine hydrochloride solution (1 mg/mL in PBS) onto working electrode, air-dry for 6 minutes
- Instrument Parameters: Set SWV frequency to 15 Hz, amplitude to 25 mV, step potential to 5 mV
- Scan Rate Optimization: Perform voltammetric scans from 0 to 1.5 V with varying scan rates (10-1000 mV/s)
- Signal Analysis: Measure peak current response at each scan rate, plot iₚ versus v¹/² to identify optimal sensitivity
- Validation: Apply optimized parameters to cocaine detection in PBS and saliva matrices
Data Interpretation: The scan rate maximizing signal-to-noise ratio while maintaining well-defined peak shape should be selected. For cocaine detection, the relationship between peak current and scan rate reveals whether the process is diffusion or adsorption-controlled, informing subsequent quantitative analysis.
Table 2: Quantitative Effects of Scan Rate on Voltammetric Parameters
| Scan Rate (V/s) | Diffusion Layer Thickness | Peak Current | Kinetic Information | Application Example |
|---|---|---|---|---|
| 0.001-0.01 (Slow) | Thick | Low | Quasi-reversible systems | Cocaine detection in saliva [86] |
| 0.01-0.1 (Medium) | Moderate | Moderate | Standard quantitative analysis | Paracetamol in pharmaceuticals [87] |
| 1-10 (Fast) | Thin | High | Transient intermediates | ORR on Au UME [85] |
| >10 (Ultrafast) | Very thin | Very high | Adsorption processes | Anthracene intermediates [85] |
The Influence of pH in Potentiometric Measurements
Fundamental Principles of pH Response
Potentiometric pH measurement relies on the development of a potential difference across a specialized glass membrane that selectively responds to hydrogen ion activity. The potential (E) follows the Nernst equation:
E = E⁰ - (2.303RT/F) × pH
Where E⁰ is the standard potential, R is the gas constant, T is temperature, and F is Faraday's constant. [84] The theoretical slope at 25°C is -59.16 mV/pH unit, providing the fundamental correlation between measured potential and solution acidity. Modern potentiometric sensors implement this principle through various configurations, including traditional glass electrodes, ion-sensitive field-effect transistors (ISFETs), and solid-contact ion-selective electrodes (SC-ISEs). [7] [84]
Advanced Potentiometric Sensor Architectures
Recent advancements in potentiometric sensors focus on enhancing stability, selectivity, and miniaturization capability:
- Solid-Contact ISEs (SC-ISEs): Replace internal solution with solid transducers (conducting polymers, carbon-based materials), improving mechanical stability and miniaturization potential [7]
- Nanocomposite Materials: Incorporate nanomaterials (metal nanoparticles, graphene, carbon nanotubes) to enhance electron transfer kinetics, sensitivity, and selectivity [7]
- 3D-Printed and Paper-Based Devices: Enable cost-effective fabrication and point-of-care applications [7]
- Wearable Potentiometric Sensors: Allow continuous monitoring of biomarkers and electrolytes in biological fluids [7]
Experimental Protocol: Potentiometric pH Sensor Calibration and Validation
Objective: Establish calibration curve and validate performance of potentiometric pH sensor for pharmaceutical quality control applications.
Materials and Equipment:
- Benchtop pH meter or potentiometric data acquisition system
- Glass pH electrode or ISFET sensor
- Standard buffer solutions (pH 4.00, 7.00, 10.00)
- Pharmaceutical samples (tablets, liquid formulations)
- Ionic strength adjustment buffer (if required)
Methodology:
- Electrode Conditioning: Soak pH electrode in storage solution or pH 7.00 buffer for 30 minutes prior to use
- Calibration Curve: Immerse electrode in standard buffers, record potential (mV) at each pH value
- Slope Determination: Plot potential versus pH, perform linear regression to determine actual slope (mV/pH)
- Sample Measurement: Immerse electrode in pharmaceutical sample (dissolved in appropriate solvent), record stable potential reading
- pH Calculation: Convert sample potential to pH using established calibration curve
- Validation: Compare results with reference method (if available), calculate accuracy and precision
Data Interpretation: The electrode slope should be 95-102% of theoretical Nernstian value (-56 to -61 mV/pH at 25°C). Sample measurements should demonstrate high reproducibility (RSD < 2%) and accuracy relative to reference values.
Comparative Experimental Toolkit
Research Reagent Solutions for Voltammetry and Potentiometry
Table 3: Essential Research Reagents and Their Functions
| Reagent/Material | Function | Application Example |
|---|---|---|
| Screen-printed electrodes | Disposable, reproducible electrode platforms | Cocaine detection in saliva [86] |
| Cocaine hydrochloride | Target analyte for forensic and clinical testing | Voltammetric sensor development [86] |
| Phosphate buffer saline (PBS) | Maintains constant pH and ionic strength | Electrochemical measurements in biological matrix [86] |
| Gold ultramicroelectrode (UME) | Enhanced mass transport, reduced iR drop | ORR intermediate studies [85] |
| Alizarin Red S | Electroactive polymer modifier for electrode surface | Paracetamol detection in serum [87] |
| ZnS NWs/rGO nanocomposite | Enhanced electrocatalytic activity and sensitivity | Homocysteine detection [88] |
| Ion-selective membranes | Provide selectivity for target ions | Potentiometric sensor fabrication [7] |
| Conducting polymers (e.g., PEDOT) | Solid-contact layer in SC-ISEs | Ion-to-electron transduction [7] |
Integrated Experimental Workflow
The following diagram outlines a comprehensive experimental workflow integrating both voltammetric and potentiometric approaches for pharmaceutical analysis:
Applications in Pharmaceutical and Clinical Research
Voltammetric Applications
Voltammetry's sensitivity and selectivity make it invaluable for pharmaceutical analysis. Recent developments include:
- Cocaine detection in saliva using biomolecule-free sensors with machine learning data processing, achieving 85% accuracy in distinguishing concentrations from 0 to >50 ng/mL [86]
- Paracetamol quantification in pharmaceuticals and serum using alizarin red S-modified electrodes, achieving detection limits of 1.0 nM [87]
- Homocysteine detection as a cardiovascular disease biomarker using ZnS nanowires/reduced graphene oxide modified electrodes, with detection limits of 0.003 μM [88]
Potentiometric Applications
Potentiometry addresses critical needs in pharmaceutical quality control and clinical monitoring:
- Therapeutic Drug Monitoring (TDM) of pharmaceuticals with narrow therapeutic indices, enabling personalized dosing regimens [7]
- Electrolyte imbalance detection in hospitalized patients, where abnormalities correlate with increased mortality and morbidity [7]
- Continuous monitoring via wearable sensors for electrolytes and pharmaceuticals in biological fluids [7]
Scan rate in voltammetry and pH in potentiometry represent fundamental parameters that dictate the sensitivity, selectivity, and application scope of these complementary electrochemical techniques. Voltammetric scan rate controls mass transport, electron transfer kinetics, and detection capability for transient species, while pH directly determines the equilibrium potential in potentiometric measurements. For drug development professionals and researchers, mastering these parameters enables optimized analytical protocols for diverse applications ranging from illicit drug detection to therapeutic drug monitoring. Future directions include increased integration of machine learning for data analysis, development of novel nanocomposite materials for enhanced sensitivity, and miniaturization of systems for point-of-care diagnostic applications. The continuing evolution of both techniques promises enhanced capabilities for chemical measurement and biomedical analysis.
Strategies for Calibration and Managing Signal Drift
In electrochemical research, the fundamental principles governing signal generation differ significantly between techniques that measure current and those that measure potential. Voltammetry, an amperometric technique, involves applying a potential and measuring the resulting current from redox reactions. In contrast, potentiometry measures the potential difference between two electrodes under conditions of negligible current flow, which provides a direct readout of ion activity [7] [89]. This distinction is critical when addressing calibration and signal drift. Potentiometric sensors are particularly valued for their high selectivity, suitability for miniaturization, and low power consumption, making them excellent candidates for embedded systems and wearable devices [7]. However, their long-term stability is heavily influenced by the potential stability of the reference electrode and the integrity of the ion-selective membrane [7]. Signal drift—the gradual change in sensor output despite a constant analyte concentration—presents a major challenge for the deployment of reliable, continuous monitoring systems, especially in clinical and pharmaceutical applications where precision is paramount.
Comparative Framework: Drift and Calibration in Voltammetry vs. Potentiometry
The strategies for managing drift and the approach to calibration are inherently shaped by the underlying measurement technique. The table below summarizes the core differences.
Table 1: Comparison of Drift and Calibration in Voltammetry and Potentiometry
| Aspect | Voltammetry (Current Measurement) | Potentiometry (Potential Measurement) |
|---|---|---|
| Primary Signal | Current (A) from faradaic reactions [89]. | Potential (V, EMF) from ion activity gradient [7]. |
| Main Drift Sources | - Fouling of electrode surface.- Depletion of electroactive species.- Changes in electrode catalytic activity [89]. | - Instability of the reference electrode potential.- Leaching of membrane components (ionophore, additives).- Water layer formation at solid-contact interfaces [7]. |
| Typical Calibration | Frequent calibration required due to surface fouling and degradation. Often involves a multi-point standard curve of current vs. concentration [89]. | Calibration can be stable for extended periods if the membrane and reference electrode are well-formulated. Based on the Nernstian or Nikolsky-Eisenman equation [7]. |
| Power Consumption | Higher, due to applied potential that drives current flow [7]. | Very low, as it measures potential at zero current flow [7]. |
A key development in potentiometry is the solid-contact ion-selective electrode (SC-ISE), which eliminates the inner filling solution of traditional electrodes. In SC-ISEs, a solid-contact layer acts as an ion-to-electron transducer, converting the ionic signal from the ion-selective membrane (ISM) into an electronic signal [7]. The stability of this interface is paramount. Two primary mechanisms govern its response: the redox capacitance mechanism and the electric-double-layer (EDL) capacitance mechanism [7]. The choice of transducer material directly impacts which mechanism dominates and, consequently, the sensor's susceptibility to drift. The following diagram illustrates the structure and signal transduction pathways in a solid-contact potentiometric sensor.
Calibration Methodologies for Potentiometric Sensors
A robust calibration protocol is the first line of defense against inaccurate measurements caused by signal drift. The goal is to establish a stable relationship between the measured potential (E) and the logarithm of the target ion's activity (log a_i).
Standard Calibration Techniques
- Multi-Point Calibration: This is the most common method, involving the measurement of potential in a series of standard solutions with known analyte concentrations. A plot of E vs. log a_i should yield a linear relationship (the Nernstian slope). The calibration curve is used to determine the unknown concentration in a sample. This should be performed regularly to account for any drift in the sensor's slope or standard potential (E°).
- Standard Addition Method: This technique is useful for analyzing complex sample matrices where the ionic background might differ significantly from calibration standards. A known volume of a standard analyte solution is added to the sample, and the change in potential is used to calculate the original sample concentration. This method helps account for matrix effects that can influence the sensor's response.
Advanced and Automated Calibration
For continuous monitoring applications, such as wearable sensors for therapeutic drug monitoring (TDM) or critical electrolyte analysis, manual calibration is not feasible [7]. Advanced strategies include:
- Integrated Calibration Fluidics: Microfluidic systems that automatically deliver calibration standards to the sensor at predefined intervals.
- Drift Compensation Algorithms: Software algorithms that model the drift based on the sensor's known behavior and automatically correct the output signal in real-time.
Experimental Protocols for Managing Signal Drift
The following protocols provide a detailed methodology for fabricating and characterizing a stable solid-contact potentiometric sensor, with a focus on minimizing drift.
Protocol: Fabrication of a Solid-Contact ISE with High Capacitance
Objective: To prepare a stable solid-contact ion-selective electrode using a high-capacitance nanomaterial transducer to minimize potential drift.
Materials and Reagents: Table 2: Essential Research Reagent Solutions for SC-ISE Fabrication
| Item | Function/Description |
|---|---|
| Glassy Carbon or Gold Electrode | Provides a stable, conductive substrate. |
| Graphene, Carbon Nanotubes, or MXenes | High-surface-area nanomaterials used as the solid-contact transducer to provide high capacitance and stable potential [7]. |
| Conducting Polymer (e.g., PEDOT:PSS) | Ion-to-electron transducer; can be used alone or in composites with nanomaterials to enhance stability [7]. |
| Ion-Selective Membrane (ISM) Cocktail | Contains the ionophore (selective agent), ionic sites (lipophilic salt), plasticizer (for PVC membranes), and polymer matrix (e.g., PVC) [7]. |
| Tetrahydrofuran (THF) | Volatile solvent used to dissolve and cast the PVC-based ISM. |
| Standard Solutions of Target Ion | Used for sensor calibration and performance evaluation. |
| Inner Electrolyte Solution (for LC-ISE only) | Contains a fixed concentration of the target ion for liquid-contact ISEs; not used in SC-ISEs [7]. |
Procedure:
- Electrode Pretreatment: Polish the solid electrode substrate (e.g., 3 mm glassy carbon) with successive grades of alumina slurry (e.g., 1.0, 0.3, and 0.05 µm) on a microcloth. Rinse thoroughly with deionized water and sonicate for 1-2 minutes to remove adsorbed particles.
- Transducer Layer Deposition: Deposit the solid-contact layer onto the polished electrode.
- Option A (Nanomaterial Dispersion): Cast a known volume (e.g., 5-10 µL) of a well-dispersed solution of graphene or carbon nanotubes onto the electrode surface and allow it to dry under ambient conditions.
- Option B (Electropolymerization): For conducting polymers, immerse the electrode in a monomer solution and apply a constant potential or use cyclic voltammetry to deposit a polymer film.
- Ion-Selective Membrane Casting: Prepare the ISM cocktail by dissolving the required amounts of ionophore, ionic additive, polymer, and plasticizer in THF. Pipette a fixed volume (e.g., 50-100 µL) of this cocktail onto the solid-contact layer. Allow the THF to evaporate slowly, covered for 24 hours, to form a homogeneous membrane.
- Conditioning and Calibration: Condition the prepared SC-ISE in a solution of the target ion (e.g., 0.01 M) for several hours or overnight to establish a stable equilibrium potential. Calibrate the sensor using a series of standard solutions (e.g., from 10⁻⁵ M to 10⁻¹ M).
Protocol: Quantifying Potential Drift via Chronopotentiometry
Objective: To evaluate the long-term stability of a solid-contact ISE by measuring its potential drift over time in a constant background solution.
Procedure:
- Setup: Place the prepared SC-ISE and a stable reference electrode (e.g., Ag/AgCl) in a stirred, fixed-concentration solution of the target ion (e.g., 0.01 M).
- Measurement: Connect the electrodes to a high-impedance potentiometer or data acquisition system. Measure and record the potential (E) at regular intervals (e.g., every second) for a prolonged period (e.g., 1-24 hours). Ensure the experimental setup is free from vibrations and temperature fluctuations.
- Data Analysis: Plot the recorded potential versus time. The potential drift is typically reported as the average change in potential per unit time (e.g., µV/h or mV/h) over a specific period, often calculated from the slope of a linear regression of the E vs. time plot.
Advanced Material Strategies to Mitigate Signal Drift
The choice of materials in the solid-contact layer is a primary factor in combating signal drift. The following workflow outlines the decision process for selecting and evaluating materials to enhance sensor stability.
Key Material Solutions:
- Conducting Polymers (CPs): Materials like poly(3,4-ethylenedioxythiophene) (PEDOT) act as excellent ion-to-electron transducers due to their mixed ionic and electronic conductivity, which helps stabilize the potential at the ISM/transducer interface [7].
- Carbon-Based Nanomaterials: Graphene, carbon nanotubes, and mesoporous carbon provide a high double-layer capacitance, which acts as an internal reservoir of charge, effectively buffering against potential changes in the sample solution and reducing drift [7].
- Nanocomposites: The most advanced strategy involves creating composites that synergize the benefits of different materials. For example, combining the high capacitance of MoS₂ nanoflowers with the magnetic properties of Fe₃O₄ nanoparticles can prevent structural collapse and further enhance capacitance [7]. Similarly, tubular gold nanoparticles modified with tetrathiafulvalene (Au-TTF) have been used to create potassium-selective electrodes with high capacitance and great potential stability [7].
Choosing the Right Tool: A Direct Comparison for Analytical Validation
Electroanalytical techniques are indispensable in modern chemical analysis, offering powerful tools for detecting and quantifying a vast array of analytes. This whitepaper provides an in-depth technical comparison between two foundational electrochemical methods: voltammetry, which measures current as a function of applied potential, and potentiometry, which measures potential difference under conditions of negligible current. The core distinction lies in their measured electrical signals—current versus potential—which fundamentally influences their sensitivity, detection limits, dynamic range, and optimal application domains [7] [36].
Sustained research and technological advancements continue to propel both techniques forward. Innovations such as nanomaterial-modified electrodes, solid-contact ion-selective electrodes, and integration with microfluidic systems have significantly enhanced their analytical performance [7] [11] [90]. This guide offers a detailed, data-driven comparison tailored for researchers, scientists, and drug development professionals, focusing on the capabilities and limitations of each method within the context of pharmaceutical and biomedical research.
Fundamental Principles and Signaling Pathways
The operational principles of voltammetry and potentiometry dictate their respective signaling pathways and analytical characteristics. The schematic workflow below illustrates the core processes for each technique.
Voltammetry Pathway: Voltammetry employs a three-electrode system (working, counter, and reference electrodes). A controlled potential is applied to the working electrode, driving the oxidation or reduction (redox) of electroactive analytes. This Faradaic process results in electron transfer, generating a measurable current directly proportional to the analyte concentration in the sample. The applied potential can be swept linearly (as in Cyclic Voltammetry, CV) or applied in pulses (as in Differential Pulse Voltammetry, DPV, or Square Wave Voltammetry, SWV) to enhance sensitivity and discrimination against capacitive currents [11] [36].
Potentiometry Pathway: Potentiometry operates under zero-current conditions using a two-electrode system comprising an ion-selective electrode (ISE) and a reference electrode. The key component is the ion-selective membrane, which contains an ionophore that selectively binds to the target ion. This selective interaction generates a membrane potential. The measured potential difference (electromotive force, EMF) between the two electrodes relates to the logarithm of the target ion's activity in the sample via the Nernst equation [7] [91]. This relationship makes potentiometry inherently selective for ionic species.
Quantitative Performance Comparison
The following tables summarize the typical and state-of-the-art performance metrics for voltammetry and potentiometry, based on recent research advancements.
Table 1: Performance Metrics for Voltammetric Techniques
| Analyte | Technique | Linear Dynamic Range | Detection Limit | Key Electrode Modification | Application Context |
|---|---|---|---|---|---|
| 2-Nitrophenol [76] | SWV | 9.9 nM - 603 µM | 2.92 nM | 2-Amino Nicotinamide / Glassy Carbon | Environmental Monitoring |
| Phosphate [92] | SWV | 10 µM - 100 µM | 1.15 µM | CuPhthalocyanine/MWCNTs | Water Quality |
| Phosphate [92] | EIS | 0.001 µM - 100 µM | 0.13 nM | CuPhthalocyanine/MWCNTs | Water Quality |
| Estrogens (Total) [93] | DPV | 15.35 - 134.55 µM | 0.08 µM (for EE2) | Boron-Doped Diamond | Environmental Monitoring |
| Bioactive Compounds [11] | Various | Picogram levels | Sub-nanomolar | Nanomaterials (e.g., Graphene, AuNPs) | Medical Diagnostics |
Table 2: Performance Metrics for Potentiometric Techniques
| Analyte | Electrode Type | Linear Dynamic Range | Detection Limit | Key Ionophore/Membrane | Application Context |
|---|---|---|---|---|---|
| Lead (Pb²⁺) [94] | Coated Graphite PVC | 1.0x10⁻⁷ - 1.0x10⁻¹ M | 7.5x10⁻⁸ M | ZMTE-MOF | Industrial Effluents |
| Lead (Pb²⁺) [91] | Solid-Contact ISE | 10⁻¹⁰ - 10⁻² M | 10⁻¹⁰ M | Nanomaterials/Conducting Polymers | Environmental Monitoring |
| Various Ions [7] | Solid-Contact ISE | Wide range | Low µM to nM | Nanocomposites, Conducting Polymers | Clinical/Biomedical |
| Phosphate (HPO₄²⁻) [95] | Polymer Membrane | 32 µM - 100 mM | 10 µM | Poly-TUS (Uranyl-Salophen) | Aqueous Solution |
Comparative Analysis of Performance Data
Sensitivity and Detection Limits: Voltammetry generally achieves superior (lower) detection limits, often extending into the nanomolar and picomolar range, especially when paired with advanced nanomaterials and pulse techniques like SWV and DPV [76] [11]. This high sensitivity stems from its foundation on current measurement, which can be amplified by electrocatalytic materials. Potentiometry, based on potential measurement, typically offers detection limits in the nanomolar to micromolar range, though state-of-the-art solid-contact ISEs with high-capacitance materials can reach sub-nanomolar levels [7] [91].
Dynamic Range: Potentiometry excels in providing an exceptionally wide dynamic range, often spanning 5 to 8 orders of magnitude of concentration (e.g., from 10⁻¹⁰ M to 10⁻² M) [91]. This is a direct consequence of the logarithmic relationship between potential and ion activity described by the Nernst equation. Voltammetric methods usually have a narrower linear dynamic range, though it is still sufficiently broad for most analytical applications.
Selectivity and Applicability: The selectivity of potentiometry is engineered through the ionophore embedded in the membrane, making it ideal for directly determining specific ionic activities (e.g., K⁺, Na⁺, Pb²⁺, Ca²⁺) in complex clinical and environmental samples [7] [94] [91]. Voltammetry's selectivity can be tailored through the choice of applied potential, electrode material, and chemical modification, enabling the detection of a wider variety of electroactive species, including organic molecules, neurotransmitters, and pharmaceuticals [76] [11] [36].
Detailed Experimental Protocols
To illustrate the practical implementation of these techniques, here are detailed methodologies for representative assays from recent literature.
Voltammetric Protocol: Determination of 2-Nitrophenol
This protocol outlines the sensitive detection of an environmental pollutant using a modified electrode and optimized square-wave voltammetry [76].
1. Electrode Modification: Prepare a 2-Amino Nicotinamide (2-AN) modified Glassy Carbon (GC) electrode.
- Procedure: Polish the bare GC electrode with alumina slurry, rinse with water, and dry. Perform electrochemical polymerization by cycling the electrode potential in a solution containing 1.0 mM 2-AN and 0.1 M H₂SO₄. The optimum number of deposition cycles for this sensor was determined to be 5. Characterize the modified surface using Scanning Electron Microscopy (SEM) and Fourier Transform Infrared Spectroscopy (FTIR) to confirm successful modification [76].
2. Optimization of SWV Parameters: Use Response Surface Methodology (RSM) to find the optimal signal response.
- Procedure: Design experiments using a Box-Behnken design to vary and optimize three key SWV parameters simultaneously: pulse amplitude, frequency, and potential step. This statistical approach minimizes the number of experiments required to find the optimal combination that yields the maximum reduction peak current for 2-Nitrophenol (2-NP) [76].
3. Sample Analysis and Quantification:
- Supporting Electrolyte: Use Britton-Robinson (BR) buffer solution at pH 1.01.
- Procedure: Place the modified 2-AN/GC electrode into the buffer solution containing the sample/standard. Record the Square Wave Voltammogram. The reduction peak of 2-NP will be observed. Use this peak for quantitative analysis.
- Calibration: Construct a calibration curve by plotting the reduction peak current against the concentration of 2-NP. The sensor exhibits two linear ranges: 9.9 nM - 52.5 µM and 52.5 µM - 603 µM, with a Limit of Detection (LOD) of 2.92 nM [76].
Table 3: Research Reagent Solutions for Voltammetric 2-NP Detection
| Reagent / Material | Function / Role in the Experiment |
|---|---|
| Glassy Carbon (GC) Electrode | Provides a stable, inert, and renewable conductive surface for electron transfer and modification. |
| 2-Amino Nicotinamide (2-AN) | Electroactive monomer that, upon electropolymerization, forms a film that pre-concentrates the analyte and enhances electron transfer. |
| Britton-Robinson (BR) Buffer | Maintains a consistent and optimal proton activity (pH 1.01) for the electrochemical reduction of 2-NP. |
| Sulfuric Acid (H₂SO₄) | Provides an acidic medium necessary for the electrochemical polymerization of the 2-AN modifier. |
| 2-Nitrophenol (2-NP) Standard | The target analyte of interest, an environmental pollutant. |
Potentiometric Protocol: Determination of Lead Ions (Pb²⁺)
This protocol describes the construction and use of a highly selective potentiometric sensor for lead ions using a synthesized Metal-Organic Framework (MOF) as an ionophore [94].
1. Ionophore Synthesis and Electrode Preparation:
- Ionophore (ZMTE-MOF) Synthesis: Synthesize the MOF by dissolving 4-methyl-1,2,4-triazole-3-thiol ligand in a methanol/ethylenediamine mixture. Slowly add this solution to a methanolic solution of ZnBr₂ under stirring. Stir the resulting clear solution at 40°C for 24 hours to form crystals [94].
- Membrane and Electrode Fabrication: Prepare a membrane cocktail with the composition: 6% ZMTE-MOF (ionophore), 2% sodium tetraphenylborate (NaTPB, additive), 32% PVC (polymer matrix), and 60% nitrobenzene (plasticizer/solvent). Coat a graphite rod electrode with this cocktail and allow the solvent to evaporate, forming a selective membrane [94].
2. Potentiometric Measurement:
- Cell Assembly: Use the prepared Pb²⁺-selective electrode as the working electrode and a traditional Ag/AgCl electrode as the reference.
- Procedure: Immerse the electrode pair in standard or sample solutions with stirring. Measure the potential difference (EMF) under zero-current conditions. Allow the reading to stabilize (response time ~5 seconds).
- Calibration: Measure the potential across a series of standard Pb²⁺ solutions. Construct a calibration curve by plotting the measured EMF (mV) versus the logarithm of Pb²⁺ activity. The sensor achieves a Nernstian slope of 30.3 mV/decade across a linear range of 1.0×10⁻⁷ to 1.0×10⁻¹ M, with an LOD of 75 nM [94].
3. Selectivity and Real Sample Validation:
- Selectivity: Determine potentiometric selectivity coefficients (( K^{pot}_{Pb,J} )) using the separate solution method or matched potential method to confirm the sensor's selectivity against interfering ions like Cu²⁺, Cd²⁺, and Zn²⁺ [94] [91].
- Validation: Test the sensor's performance in real samples (e.g., industrial effluents) and validate the results against a standard method like Inductively Coupled Plasma Mass Spectrometry (ICP-MS) [94].
Table 4: Research Reagent Solutions for Potentiometric Pb²⁺ Detection
| Reagent / Material | Function / Role in the Experiment |
|---|---|
| ZMTE-MOF (Zn²+, 4-methyl-1,2,4-triazole-3-thiol) | The ionophore; selectively complexes with Pb²⁺ ions, defining the sensor's selectivity. |
| Polyvinyl Chloride (PVC) | The polymer matrix that forms the bulk of the ion-selective membrane, providing structural integrity. |
| Nitrobenzene | The plasticizer; dissolves the membrane components, provides ion mobility, and influences the membrane's dielectric constant. |
| Sodium Tetraphenylborate (NaTPB) | A lipophilic ionic additive; prevents co-ion interference and improves the membrane's permselectivity. |
| Ag/AgCl Reference Electrode | Provides a stable, known reference potential against which the potential of the ISE is measured. |
Advanced Technical Considerations
Signal Enhancement and Interference Management
Voltammetry: A primary challenge is electrode fouling and interference from complex sample matrices. Strategies to mitigate this include:
- Nanomaterial Modification: Using materials like graphene, carbon nanotubes (CNTs), metal nanoparticles (e.g., Au, Ag), and metal-organic frameworks (MOFs) to increase the electroactive surface area, enhance electron transfer kinetics, and provide catalytic activity. For instance, COF/NH₂-CNT composites have been used to sensitively determine 2-NP [76] [11].
- Pulse Techniques: Utilizing SWV and DPV to minimize the contribution of capacitive charging currents, thereby enhancing the Faradaic current signal and lowering the detection limit [76] [36].
- Surface Renewal: Employing electrodes like Boron-Doped Diamond (BDD) that offer low fouling characteristics and a wide potential window, ideal for detecting compounds that require high oxidation potentials [93].
Potentiometry: The main challenges are signal drift and selectivity against interfering ions.
- Solid-Contact Transducers: Replacing traditional liquid-filled electrodes with solid-contact (SC) ISEs using conducting polymers (e.g., PEDOT, polyaniline) or high-surface-area carbon nanomaterials (e.g., MXenes, CNTs). This eliminates the inner filling solution, easing miniaturization and improving mechanical stability and signal stability by providing a high capacitance interface that buffers against potential drift [7] [91].
- Ionophore Design: The continuous development of novel ionophores—from classical ligands to advanced MOFs and covalent organic frameworks (COFs)—is critical for achieving high selectivity. Computational chemistry (e.g., Density Functional Theory) is increasingly used to screen and design ionophores with high affinity for the target ion [94].
Emerging Trends and Future Outlook
The convergence of electrochemistry with other disciplines is shaping the future of both voltammetry and potentiometry.
- Miniaturization and Wearable Sensors: There is a strong trend towards developing miniaturized, portable, and even wearable potentiometric and voltammetric sensors for continuous, on-body monitoring of electrolytes, pharmaceuticals, and biomarkers in sweat, interstitial fluid, or other biofluids [7] [36].
- Advanced Manufacturing: 3D printing is emerging as a powerful tool for the rapid prototyping and fabrication of customized electrochemical sensors and fluidic components, decreasing development time and cost [7].
- Integration with Microfluidics and AI: Coupling electrochemical sensors with microfluidic systems allows for automated, high-throughput analysis with minimal sample consumption. Furthermore, the integration of Artificial Intelligence (AI) and machine learning is poised to revolutionize data interpretation, sensor calibration, and the identification of complex patterns in electrochemical data [36] [90].
- Sustainable Materials: The development of environmentally friendly and biodegradable materials for sensor construction is an area of growing interest, aligning with the principles of green chemistry [11].
The choice between voltammetry and potentiometry is not a matter of one technique being superior to the other, but rather of selecting the right tool for the specific analytical problem.
Voltammetry is the method of choice when the requirement is for extremely low detection limits (nanomolar to picomolar) for a wide range of electroactive species, including organic molecules, pharmaceuticals, and heavy metals. Its strength lies in its high sensitivity and the rich mechanistic information it can provide.
Potentiometry excels in the direct, selective, and rapid measurement of ionic activity in complex matrices like blood, urine, or wastewater. Its key advantages are a very wide dynamic range, operational simplicity, portability, and low power consumption, making it ideal for point-of-care testing and continuous environmental monitoring.
The ongoing innovation in nanomaterials, electrode design, and data science will further blur the lines between these techniques, leading to the development of hybrid, intelligent, and connected sensing systems that will profoundly impact pharmaceutical research, clinical diagnostics, and environmental protection.
The quantitative analysis of colored, turbid, or low-volume samples presents significant challenges for optical analytical techniques due to light absorption, scattering, or limited sample availability. Within the context of electrochemical research, a fundamental distinction exists between techniques that measure current under controlled potential (voltammetry) and those that measure potential at zero current (potentiometry). This whitepaper provides an in-depth technical examination of how these electrochemical methods are suited for analyzing complex matrices, with a specific focus on their operational principles, methodological adaptations, and practical applications in pharmaceutical and environmental research. Voltammetry, which involves applying a controlled potential and measuring the resulting current, offers exceptional sensitivity for trace-level analysis of electroactive species, while potentiometry, which measures the potential of electrochemical cells at zero current, provides selective determination of ionic activities even in highly colored or turbid media where optical methods fail [1]. For researchers in drug development, understanding these distinctions is critical for selecting the appropriate analytical technique to overcome matrix interferences, minimize sample preparation, and obtain reliable analytical data from challenging samples.
Theoretical Framework: Current vs. Potential Measurement
Fundamental Principles of Voltammetry and Potentiometry
Electrochemical analysis techniques are broadly categorized based on whether they control potential and measure current (voltammetry) or measure potential at zero current (potentiometry). This fundamental distinction dictates their applicability to different sample types and matrices.
Voltammetry is a dynamic technique where the current passing through an electrochemical cell is measured as a function of the applied potential. The resulting voltammogram provides both qualitative and quantitative information about the analyte. The three-electrode system—consisting of a working electrode (WE), reference electrode (RE), and counter electrode (CE)—is crucial for precise potential control [1]. Key voltammetric techniques include:
- Cyclic Voltammetry (CV): Potential is scanned in forward and reverse directions to study reaction mechanisms and kinetics.
- Differential Pulse Voltammetry (DPV) and Square Wave Voltammetry (SWV): Pulsed techniques that enhance sensitivity for trace analysis by minimizing background current.
Potentiometry measures the potential difference between two electrodes at zero current conditions. This potential is related to the concentration of the target ion through the Nernst equation [1]. The most common applications include:
- Ion-Selective Electrodes (ISEs): Designed to respond selectively to specific ions (e.g., F−, Na+, K+).
- Potentiometric Titrations: The endpoint is determined by monitoring potential changes rather than using visual indicators.
Comparative Advantages for Complex Samples
The table below summarizes the fundamental differences between these approaches and their implications for analyzing complex matrices:
Table 1: Core Principles and Advantages of Voltammetry and Potentiometry
| Aspect | Voltammetry | Potentiometry |
|---|---|---|
| Measured Quantity | Current as function of applied potential [1] | Potential at zero current [1] |
| Primary Information | Qualitative & quantitative (identity & concentration) [1] | Quantitative (ion activity/ concentration) [1] |
| Sensitivity | Excellent for trace analysis (pulsed techniques) [1] | Moderate (suitable for routine ion measurements) [1] |
| Selectivity | Achieved through potential control & electrode modification | Inherent through ion-selective membranes [1] |
| Sample Volume | Adaptable to micro volumes with specialized cells | Typically requires larger volumes, but flow systems enable miniaturization [96] |
Diagram 1: Electrochemical Approaches for Complex Samples
Methodological Approaches for Challenging Matrices
Analysis of Colored and Turbid Samples
Turbid and colored samples present significant challenges for optical analytical methods due to light scattering and absorption. However, electrochemical techniques are largely unaffected by these optical interferences, making them particularly suitable for such matrices.
Overcoming Optical Interferences: While turbidimetry (measuring light absorption) and nephelometry (measuring scattered light) are used for turbid samples, they require special configurations to minimize multiple scattering events [97] [98]. In contrast, electrochemical methods like voltammetry and potentiometry are inherently immune to these interferences because they rely on electron transfer rather than light transmission. This advantage is particularly valuable for:
- Environmental samples: Suspended particles cause turbidity in natural waters.
- Biological fluids: Proteins and cellular components create turbidity.
- Industrial slurries: High particle concentrations scatter light.
Experimental Considerations:
- For voltammetry in turbid matrices, electrode fouling can be mitigated by using pulsed techniques or modified electrode surfaces.
- Potentiometric measurements with ion-selective electrodes remain accurate in colored solutions where photometric methods would fail [96].
- Flow-based systems can automate sample handling and minimize matrix effects [96].
Low-Volume and Microscale Analysis
Pharmaceutical and clinical applications often involve limited sample volumes, requiring adaptations of standard electrochemical techniques.
Microelectrodes and Miniaturized Systems: The use of microelectrodes enables measurements in small volumes (microliters) while offering advantages such as reduced ohmic drop and enhanced mass transport. Recent advancements include:
- Lab-on-a-chip devices integrating microfluidic sample handling with electrochemical detection.
- Portable handheld instruments for field analysis of limited samples [99].
- Flow injection analysis with low-volume detection cells [96].
Methodological Adaptations:
- Standard addition methods in flow systems enable automatic multiple additions with minimal sample consumption [96].
- Miniaturized potentiometric sensors for in-vivo or point-of-care applications.
- Arrays of microelectrodes for high-throughput screening in drug development.
Experimental Protocols and Applications
Detailed Methodologies for Complex Sample Analysis
Protocol 1: Voltammetric Analysis of Trace Metals in Turbid Waters
Principle: Differential pulse voltammetry (DPV) enhances sensitivity for trace metal analysis by minimizing charging current. The technique is unaffected by sample color or turbidity [1].
Procedure:
- Sample Preparation: Acidify water sample to pH 2.0 with ultrapure nitric acid. For highly turbid samples, degas with nitrogen for 5 minutes without filtration.
- Instrument Parameters:
- Technique: Differential Pulse Voltammetry
- Working Electrode: Hanging Mercury Drop Electrode (HMDE) or modified carbon electrode
- Potential Range: Species-dependent (e.g., -1.2 to 0 V for Cd, Pb, Cu)
- Pulse Amplitude: 50 mV
- Step Potential: 2 mV
- Scan Rate: 10 mV/s
- Calibration: Use standard addition method with at least three additions to account for matrix effects.
- Measurement: Record voltammograms after 30-second deposition at optimal potential with stirring.
Data Interpretation: Peak currents are proportional to concentration. Peak potentials identify specific metals. The method achieves detection limits of 0.1-1 μg/L for heavy metals [100] [1].
Protocol 2: Potentiometric Determination of Fluoride in Food Samples
Principle: Fluoride-selective electrode based on LaF₃ crystal membrane measures fluoride activity via potential difference, unaffected by sample color [96].
Procedure:
- Sample Preparation:
- For sea salt: Dissolve 5 g in 50 mL deionized water with Total Ionic Strength Adjustment Buffer (TISAB).
- For coffee beverages: Centrifuge at 4000 rpm for 10 minutes, then dilute supernatant with TISAB (1:1).
- Flow System Configuration:
- Use merging zones approach for standard additions [96].
- Peristaltic pump with flow rate: 1.5 mL/min.
- Mixing coil: 100 cm.
- Standard Additions:
- Automatically add three different standard concentrations to sample.
- Measure potential after each addition until stable reading (±0.2 mV over 10s).
- Calibration: Use Gran's plot method for data evaluation at low concentration levels.
Performance: This approach achieves limit of quantification of 5×10⁻⁶ mol L⁻¹, enabling determination of fluoride at levels below the conventional LOQ of the potentiometric detector [96].
Quantitative Comparison of Techniques
The table below provides a detailed comparison of technique performance for different sample types:
Table 2: Performance Characteristics for Complex Sample Analysis
| Technique | Sample Type | Linear Range | Detection Limit | Matrix Tolerance | Analysis Time |
|---|---|---|---|---|---|
| Differential Pulse Voltammetry | Turbid waters | 0.5-100 μg/L (Cd, Pb) | 0.1 μg/L | High (unaffected by color/turbidity) | 5-10 min [1] |
| Square Wave Voltammetry | Pharmaceutical compounds | 10⁻⁸-10⁻⁵ M | 5×10⁻⁹ M | Moderate (may require cleanup) | 3-5 min [1] |
| Fluoride ISE | Food samples (salt, coffee) | 5×10⁻⁶-10⁻¹ M | 2×10⁻⁶ M | High (unaffected by color) | 8 samples/h (with flow system) [96] |
| pH ISE | Colored beverages | 2-12 pH units | 0.01 pH units | High (unaffected by color) | <1 min [1] |
| Turbidimetry (reference) | Bacterial suspensions | 0-100 NTU | 0.05 NTU | Low (affected by color) | <2 sec [99] |
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful analysis of complex samples requires appropriate selection of reagents, electrodes, and instrumentation. The following table details essential materials and their functions:
Table 3: Essential Research Reagents and Materials
| Item | Function/Application | Technical Specifications | Example Use Cases |
|---|---|---|---|
| Ion-Selective Electrodes | Potentiometric detection of specific ions | Fluoride ISE: LaF₃ crystal membrane; pH ISE: glass membrane | Fluoride in food samples [96]; pH in colored solutions [1] |
| Working Electrodes | Voltammetric sensing platform | Glassy carbon, HMDE, modified carbon electrodes | Trace metal detection [100]; drug compound analysis [1] |
| TISAB Buffer | Ionic strength adjustment & pH control | Contains CDTA to complex Al³⁺, Fe³⁺; acetate buffer pH 5.0-5.5 | Fluoride determination in complex matrices [96] |
| Supporting Electrolyte | Provide conductivity, fix ionic strength | 0.1 M KNO₃, KCl, or acetate buffer pH 4.5 | Voltammetric analysis of metal ions [100] |
| Formazine Standards | Turbidity calibration | Prepared from hydrazine sulfate & hexamethylenetetramine | Reference method for turbid samples [98] |
| Standard Solutions | Calibration & standard additions | Certified reference materials traceable to NIST | Quantification of analytes in unknown samples [96] |
Advanced Applications and Workflow Integration
Integrated Workflow for Complex Sample Analysis
The analysis of challenging matrices often requires a systematic approach that combines multiple techniques or specialized methodologies. The following diagram illustrates a decision workflow for selecting and applying appropriate electrochemical methods:
Diagram 2: Analysis Workflow for Complex Matrices
Advanced Applications in Pharmaceutical Research
Electrochemical methods offer unique advantages for pharmaceutical analysis where samples may be colored, turbid, or available only in limited quantities.
Drug Metabolism Studies:
- Voltammetric profiling of electroactive metabolites in biological fluids.
- Potentiometric monitoring of ionic species in cellular assays.
Formulation Analysis:
- Direct measurement of active ingredients in colored syrups or suspensions.
- Dissolution testing without interference from suspended particles.
Quality Control of Biologics:
- Monitoring critical ions in fermentation broths using potentiometry.
- Turbidity assessment of protein solutions complemented with electrochemical analysis.
The analysis of colored, turbid, and low-volume matrices requires analytical techniques that are immune to optical interferences and adaptable to challenging conditions. Voltammetry and potentiometry offer complementary approaches for such applications, with voltammetry excelling in trace analysis of electroactive species and potentiometry providing selective determination of ionic activities. The methodologies and protocols detailed in this technical guide provide researchers with robust frameworks for implementing these electrochemical techniques in pharmaceutical development, environmental monitoring, and food analysis. By selecting the appropriate technique based on sample characteristics and analytical requirements, scientists can obtain reliable data from even the most challenging matrices without extensive sample preparation or compromise in analytical performance.
Electroanalytical techniques have become indispensable in modern pharmaceutical analysis, offering highly sensitive, selective, and cost-effective methods for drug quantification. This whitepaper provides a comprehensive technical comparison between two principal electrochemical methodologies: voltammetry, which measures current as a function of applied potential, and potentiometry, which measures potential under conditions of zero current. The distinction between current measurement in voltammetry and potential measurement in potentiometry represents a fundamental divergence in operational principle and application scope, forming the core investigation of this document. As the pharmaceutical industry increasingly adopts Quality by Design (QbD) principles and seeks sustainable analytical methods, understanding the specific capabilities, limitations, and appropriate application contexts of these techniques becomes paramount for researchers, scientists, and drug development professionals [101] [36].
The following sections present an in-depth comparison of key analytical figures of merit, detailed experimental protocols, and a scientific resource toolkit to guide method selection and implementation in both research and quality control environments.
Comparative Analytical Performance Data
The analytical performance of voltammetric and potentiometric methods is quantified through standardized figures of merit. The data below, compiled from recent pharmaceutical applications, demonstrates that both techniques offer excellent sensitivity and wide linear dynamic ranges, suitable for various pharmaceutical matrices from bulk active pharmaceutical ingredient (API) quantification to therapeutic drug monitoring in biological fluids.
Table 1: Key Figures of Merit for Voltammetric Pharmaceutical Assays
| Analyte | Technique | Linear Range | Detection Limit | Electrode | Matrix | Citation |
|---|---|---|---|---|---|---|
| Ascorbic Acid (AA) | DPV | 1.7 – 60.5 mg L⁻¹ | 0.5 mg L⁻¹ | Screen-Printed Carbon | Pharmaceutical, Environmental | [102] |
| Paracetamol (PA) | DPV | 0.6 – 40.0 mg L⁻¹ | 0.2 mg L⁻¹ | Screen-Printed Carbon | Pharmaceutical, Environmental | [102] |
| Dextromethorphan (DX) | DPV | 0.9 – 8.4 mg L⁻¹ | 0.3 mg L⁻¹ | Screen-Printed Carbon | Pharmaceutical, Environmental | [102] |
| Caffeine (CF) | DPV | 1.8 – 22.0 mg L⁻¹ | 0.5 mg L⁻¹ | Screen-Printed Carbon | Pharmaceutical, Environmental | [102] |
| Methimazole | SWV | 1 – 700 μmol L⁻¹ | 0.5 μmol L⁻¹ | Not Specified | Tablet Formulation | [103] |
Table 2: Key Figures of Merit for Potentiometric Pharmaceutical Assays
| Analyte | Slope (mV/decade) | Linear Range (M) | Detection Limit (M) | Sensor Type | Matrix | Citation |
|---|---|---|---|---|---|---|
| Cyclobenzaprine HCl | 57.97 ± 0.23 | 1.0×10⁻⁷ – 1.0×10⁻² | 5.62×10⁻⁸ | Ion-Selective (Graphite) | Wastewater | [104] |
| Cytarabine | 52.3 ± 1.2 | 1.0×10⁻⁶ – 1.0×10⁻³ | 5.5×10⁻⁷ | Ion-Selective (MIP) | Pharmaceutical, Serum | [105] |
| Letrozole (TBCAX-8) | 19.90 | 1.0×10⁻⁵ – 1.0×10⁻² | - | Ion-Selective (Calixarene) | Dosage Form | [106] |
| Letrozole (GNC) | 20.10 | 1.0×10⁻⁶ – 1.0×10⁻² | - | Solid Contact (Graphene) | Dosage Form | [106] |
| Letrozole (PANI) | 20.30 | 1.0×10⁻⁸ – 1.0×10⁻³ | - | Solid Contact (Polyaniline) | Plasma, Dosage Form | [106] |
Experimental Protocols
Voltammetric Protocol for Multi-Component Analysis
Voltammetric techniques, such as Differential Pulse Voltammetry (DPV), are prized for their ability to simultaneously determine multiple electroactive species in a single measurement. The following protocol outlines a validated method for the concurrent analysis of ascorbic acid, paracetamol, dextromethorphan, and caffeine using screen-printed carbon electrodes (SPCEs) [102].
- Instrumentation and Reagents: A potentiostat compatible with screen-printed electrodes is required. Use a commercial SPCE (e.g., Metrohm DropSens ref. 110) comprising carbon working and auxiliary electrodes and a silver reference electrode. Prepare a 0.1 M acetic/acetate buffer at pH 5.00 as the supporting electrolyte. Stock solutions of the target analytes should be prepared weekly: Ascorbic Acid (AA) and Paracetamol (PA) in ultrapure water, and Dextromethorphan (DX) in absolute ethanol due to its limited aqueous solubility. Store all stock solutions at 4°C.
- Electrode Preparation and Measurement: Perform all measurements in a 25 mL electrochemical cell containing the supporting electrolyte. Connect the SPCE to the potentiostat via a flexible cable. First, record repeated blank measurements in the pure buffer until a stable background current is achieved. For analysis of samples containing caffeine, apply a conditioning potential of -0.5 V for 30 seconds to desorb any previously adsorbed analyte. The DPV parameters are as follows: potential window from -0.5 V to +1.5 V, a step potential of 5 mV, a pulse amplitude of 0.1 V, a pulse time of 50 ms, and a scan rate of 0.01 V s⁻¹. Measurements can be performed without prior deoxygenation.
- Calibration and Quantification: Construct individual or simultaneous calibration curves by adding increasing concentrations of the target analytes to the buffer. Plot the peak current against the analyte concentration. The oxidative peaks for the four drugs are typically well-resolved, allowing for simultaneous quantification. For pharmaceutical formulations like granules, a sample must be accurately weighed, dissolved, and diluted with the supporting electrolyte before measurement.
Potentiometric Protocol for Ion-Selective Electrode Fabrication
Potentiometric sensors measure the equilibrium potential across a selective membrane, which is proportional to the logarithm of the target ion's activity. This protocol details the construction and calibration of a molecularly imprinted polymer (MIP)-based ion-selective electrode for the determination of cytarabine, an antileukemia drug [105].
- Synthesis of Molecularly Imprinted Polymer (MIP): The sensory element is a biomimetic receptor tailored for cytarabine. Combine the template molecule (cytarabine, 0.5 mmol) with the functional monomer (methacrylic acid, 1.5 mmol), the crosslinker (ethylene glycol dimethacrylate, 1.5 mmol), and an initiator (benzoyl peroxide, 50.0 mg) in 10 mL of acetonitrile. Sonicate the mixture for 15 minutes and purge with dry nitrogen for 15 minutes to remove dissolved oxygen. Subsequently, carry out thermal polymerization in an oil bath at 70°C for 18 hours. After polymerization, wash the resulting MIP beads with methanol and then use Soxhlet extraction with an acetic acid/methanol mixture (2:8, v/v) to thoroughly remove the template molecule. Confirm complete template removal by verifying the absence of cytarabine's UV absorbance at 277.5 nm in the eluate.
- Sensor Fabrication: To prepare the ion-selective membrane, thoroughly mix 8.8 mg of the prepared MIP beads, 66.5 mg of poly(vinyl chloride) (PVC) as the polymeric matrix, 127 mg of a plasticizer (e.g., o-nitrophenyl octyl ether, o-NPOE), and 2.2 mg of a lipophilic salt (e.g., potassium tetrakis(3,5-bis(trifluoromethyl)phenyl)borate, KTFPB) in 3 mL of tetrahydrofuran (THF). Pour this mixture into a 3-cm diameter petri dish and allow the THF to evaporate slowly at room temperature, resulting in a plasticized PVC membrane. Cut a 10-mm diameter disk from this membrane and glue it to a PVC tube using THF. Fill the electrode body with an internal solution of 10⁻³ M cytarabine hydrochloride and assemble the electrode with an Ag/AgCl reference system.
- Calibration and Measurement: Calibrate the electrode by measuring the potential in a series of standard cytarabine solutions (e.g., from 10⁻⁶ to 10⁻³ M), each prepared in a 30 mM acetate buffer at pH 3.5. Plot the measured potential (mV) against the logarithm of the cytarabine concentration. The plot should yield a linear region with a slope close to the theoretical Nernstian value. The electrode can then be used to determine unknown concentrations of cytarabine in samples such as pharmaceutical formulations or spiked human serum by interpolating the measured potential on the calibration curve.
Fundamental Principles and Workflows
The core distinction between voltammetry and potentiometry lies in what is measured: current under a controlled potential versus potential at zero current. This fundamental difference dictates their operational workflows, applications, and the information they provide.
Figure 1: Core Measurement Principles of Voltammetry and Potentiometry
Voltammetry is a dynamic technique where an applied potential drives a redox reaction, and the resulting current is measured. This current is directly proportional to the concentration of the electroactive species, such as an Active Pharmaceutical Ingredient (API), in the solution [36]. In contrast, potentiometry is a static technique performed under conditions of zero current. It measures the potential established across a selective membrane that separates the sample from a reference solution. This potential is governed by the Nernst equation and is logarithmically related to the activity (concentration) of the target ion [36] [105]. The choice of technique thus hinges on the analytical question: voltammetry is ideal for studying redox behavior and detecting multiple electroactive compounds simultaneously, while potentiometry excels in the specific, direct measurement of particular ions or ionized drug molecules.
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful implementation of electrochemical pharmaceutical assays requires specific materials and reagents. The following table details essential components and their functions for both voltammetric and potentiometric methods.
Table 3: Essential Research Reagents and Materials for Electrochemical Assays
| Category | Item | Primary Function | Example Application |
|---|---|---|---|
| Electrodes & Sensors | Screen-Printed Carbon Electrode (SPCE) | Disposable, reproducible working electrode for voltammetry. | Simultaneous determination of AA, PA, DX, CF [102]. |
| Ion-Selective Membrane | Sensory element for selective analyte recognition in potentiometry. | Cytarabine sensor using MIP beads [105]. | |
| Ag/AgCl Reference Electrode | Provides stable, reproducible reference potential. | Standard reference system in most potentiometric cells [105] [106]. | |
| Polymeric Matrix & Additives | Poly(Vinyl Chloride) (PVC) | Forms the backbone of the ion-selective membrane. | Matrix for MIP-based and conventional ISEs [105] [106]. |
| Plasticizers (e.g., o-NPOE, DOP) | Imparts flexibility and mobility to the PVC membrane; influences selectivity. | Essential component in all PVC membrane ISEs [105] [106]. | |
| Lipophilic Salt (e.g., KTFPB) | Reduces membrane resistance and improves potentiometric response. | Added to ISE membrane cocktails [105]. | |
| Chemical Modifiers | Molecularly Imprinted Polymers (MIPs) | Synthetic receptors providing high selectivity for target molecules. | Biomimetic recognition of cytarabine in ISEs [105]. |
| Nanomaterials (Graphene, PANI) | Enhance signal transduction, stability, and sensitivity in solid-contact ISEs. | Modified sensors for Letrozole detection [106]. | |
| Solvents & Buffers | Tetrahydrofuran (THF) | Solvent for casting PVC-based ion-selective membranes. | Membrane preparation for ISEs [105] [106]. |
| Supporting Electrolyte/Buffer | Carries current, defines pH, and controls ionic strength. | Acetate buffer for voltammetric drug determination [102]. |
This whitepaper has provided a detailed comparative analysis of voltammetry and potentiometry, framing them within the core research context of current versus potential measurement. The structured data and protocols demonstrate that both techniques are powerful, yet each possesses distinct strengths. Voltammetry, particularly with modern screen-printed electrodes, offers unparalleled capability for the rapid, simultaneous determination of multiple electroactive pharmaceuticals in complex matrices. Potentiometry, through advanced materials like molecularly imprinted polymers and conductive nanomaterials, provides exceptional selectivity and sensitivity for specific ions or ionizable drugs, making it ideal for therapeutic drug monitoring and routine quality control. The choice between these techniques is not a matter of superiority but of strategic application based on the analytical requirements, sample matrix, and desired information. As the field progresses, the integration of nanotechnology, artificial intelligence, and sustainable materials will further enhance the capabilities of both methods, solidifying their roles as indispensable tools in pharmaceutical research and development [36].
In the landscape of analytical chemistry, two principal electrochemical methodologies dominate quantitative analysis: voltammetry, which measures current as a function of applied potential, and potentiometry, which measures potential difference at zero current [10] [16]. Voltammetric techniques, such as cyclic voltammetry (CV) and square wave voltammetry (SWV), are renowned for their high sensitivity and ability to study electrochemical mechanisms and reaction rates [10] [107]. In contrast, potentiometry, primarily employing ion-selective electrodes (ISEs), offers exceptional selectivity for specific ions, simplicity, and suitability for continuous monitoring in complex matrices like biological fluids [7] [5]. Despite their divergent signal transduction principles—current measurement versus potential measurement—both techniques are pillars in pharmaceutical, environmental, and clinical analysis.
The critical importance of analytical method validation transcends the choice of technique. In regulated environments, such as pharmaceutical development, validation provides documented evidence that an analytical method is fit for its intended purpose, ensuring the reliability, consistency, and accuracy of data submitted for regulatory approval [108]. Parameters including precision, accuracy, and ruggedness form the bedrock of this process. This guide details the formal definitions, experimental protocols, and acceptance criteria for these core validation parameters, contextualized within a framework comparing the voltammetric and potentiometric approaches.
Core Principles: Voltammetry vs. Potentiometry
The fundamental difference between these techniques lies in their operational principles and the signals they monitor, which directly influences their application and validation.
Voltammetry is an amperometric technique where current is measured as the applied potential is varied [16] [55]. The resulting plot of current versus potential (a voltammogram) provides rich qualitative and quantitative information. The current is a faradaic current, resulting from the oxidation or reduction of an analyte at the working electrode surface. Techniques include:
- Linear Sweep Voltammetry (LSV)
- Cyclic Voltammetry (CV): Often used to evaluate antioxidant capacity by measuring oxidation potentials of compounds like phenolic acids [109].
- Square Wave Voltammetry (SWV): A pulsed technique known for its sensitivity and speed, frequently used in advanced sensor applications like electrochemical aptamer-based (EAB) sensors [107].
Potentiometry involves the measurement of the potential (voltage) of an electrochemical cell at zero current (or negligible current) [5] [16] [55]. This potential difference, measured between an indicator electrode (e.g., an Ion-Selective Electrode, ISE) and a reference electrode, is related to the logarithm of the target ion's activity by the Nernst equation. This technique is widely used for direct ion concentration measurement with applications from clinical analysis to environmental monitoring [7].
Table 1: Comparative Overview of Voltammetry and Potentiometry
| Feature | Voltammetry | Potentiometry |
|---|---|---|
| Measured Signal | Current (i) | Potential (E) |
| Controlled Signal | Potential (E) | Current (i ≈ 0) |
| Primary Output | Voltammogram (i vs. E) | Potential reading (E) |
| Key Relationship | Current ∝ concentration | Potential ∝ log(activity) |
| Common Techniques | CV, SWV, DPV, LSV | Direct Potentiometry, Potentiometric Titration |
| Typical Applications | Mechanism study, antioxidant activity, trace metal analysis, biosensors [109] [107] | pH measurement, ion concentration (Na+, K+, Cl-) in clinical/biological samples [7] |
Diagram 1: Method Validation Workflow
Essential Reagents and Materials for Electroanalytical Research
The experimental setup and required materials differ significantly between voltammetric and potentiometric methods.
Table 2: Research Reagent Solutions and Key Materials
| Item | Function/Description | Common Examples |
|---|---|---|
| Three-Electrode System | Standard setup for voltammetry: Working Electrode (WE), Reference Electrode (RE), Counter Electrode (CE) [107]. | Glassy Carbon Electrode (GCE) [109], Ag/AgCl RE, Pt CE. |
| Ion-Selective Electrode (ISE) | Indicator electrode for potentiometry; selectively binds target ion [7] [5]. | pH glass electrode, K+-selective electrode with valinomycin [110]. |
| Solid-Contact ISE (SC-ISE) | ISE without inner filling solution; uses a solid-contact layer for transduction [7]. | SC-ISEs with conducting polymers (e.g., PEDOT) or carbon-based materials. |
| Reference Electrode | Provides a stable, constant potential for measurement [5]. | Ag/AgCl, Calomel (SCE). |
| Redox Reporter | Molecule attached to biomolecules (e.g., aptamers) for voltammetric biosensing. | Methylene Blue (used in EAB sensors) [107]. |
| Ionophore | Membrane component in ISEs that selectively complexes with the target ion [7]. | Valinomycin (for K+), Hydrogen ionophore (for H+). |
| Ionic Additives | Lipophilic salts in ISE membranes to reduce membrane resistance and improve selectivity [110]. | Potassium tetrakis(4-chlorophenyl)borate. |
| Polymeric Membrane Matrix | The backbone of the sensing membrane in polymeric ISEs [110]. | Plasticized Poly(Vinyl Chloride) (PVC). |
Defining and Determining Key Validation Parameters
Accuracy
Accuracy is defined as the closeness of agreement between a test result and an accepted reference value [108]. It is a measure of exactness and is typically reported as percent recovery.
Experimental Protocol for Voltammetry (e.g., Antioxidant Assay):
- Standard Preparation: Prepare a series of standard solutions of the analyte (e.g., gallic acid) at a minimum of three concentration levels across the specified range of the method (e.g., 80%, 100%, 120% of target). Analyze each concentration in triplicate [108].
- Sample Analysis: Quantify the analyte concentration using the voltammetric method (e.g., Cyclic Voltammetry at a Glassy Carbon Electrode) [109].
- Calculation: Calculate the percent recovery for each concentration using the formula: % Recovery = (Measured Concentration / Known Concentration) × 100.
- Acceptance Criteria: Data should be collected from a minimum of nine determinations. The mean recovery should be within established limits, for example, 98-102% for a drug substance [108].
Experimental Protocol for Potentiometry (e.g., Ion Analysis):
- Standard Addition or Calibration Curve: Use standard solutions with known ion activities to construct a calibration curve (E vs. log[a]) or employ the standard addition method.
- Spiked Sample Analysis: Analyze real samples (e.g., drug product, serum) that have been spiked with known quantities of the target ion.
- Calculation and Acceptance: Determine the recovered concentration and calculate the percent recovery as above. The results should meet pre-defined acceptance criteria for the sample matrix.
Precision
Precision expresses the closeness of agreement between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions [108]. It is subdivided into three tiers:
Repeatability (Intra-assay Precision): Precision under the same operating conditions over a short interval of time [108].
- Protocol: Prepare six independent sample preparations of a single homogeneous sample at 100% of the test concentration. Analyze all six using the same method, analyst, and equipment on the same day. Report the results as % Relative Standard Deviation (%RSD).
- Example (Voltammetry): The oxidation peak potential (Ep,a) for compounds like sesamol or eugenol in CV exhibits a confidence interval of ±4 mV, demonstrating good repeatability [109].
Intermediate Precision: Precision within the same laboratory, incorporating variations like different days, different analysts, or different equipment [108].
- Protocol: Two different analysts prepare and analyze replicate sample preparations on different days, using different HPLC systems or potentiostats. The %-difference in the mean values between the two sets is calculated and subjected to statistical testing (e.g., Student's t-test).
Reproducibility (Including Ruggedness): Precision between different laboratories, as in collaborative studies [108]. Ruggedness is the degree of reproducibility of results under a variety of normal, expected conditions, such as different laboratories, analysts, instruments, and reagent lots. The term "ruggedness" is now often incorporated into the assessment of intermediate precision and reproducibility per ICH guidelines [108].
Ruggedness
As a subset of reproducibility, ruggedness is formally defined as the degree of reproducibility of test results obtained under a variety of normal, expected conditions, such as different laboratories, analysts, instruments, reagent lots, elapsed assay times, assay temperature, or days [108]. It is a measure of the method's robustness to routine operational and environmental variables.
Experimental Protocol for Ruggedness Testing: A ruggedness test is an interlaboratory study designed to identify factors that significantly affect the analytical results.
- Define Variables: Identify key variables that may impact the method (e.g., analyst, laboratory, instrument model, incubation time, buffer pH, temperature).
- Experimental Design: Use a structured experimental design (e.g., a factorial design) where these variables are deliberately altered in a controlled manner.
- Analysis: Analyze the results to determine which variables cause statistically significant changes in the analytical outcome (e.g., assay result, impurity profile).
- Outcome: The method is considered rugged with respect to a variable if the variation introduced is within the pre-defined precision limits. If a variable is found to be critical, the method procedure may need to be tightened to control that variable more strictly.
Diagram 2: Precision and Accuracy Parameters
Advanced Applications and Case Studies
Voltammetry: Interrogating Electrochemical Aptamer-Based (EAB) Sensors
EAB sensors are a revolutionary technology for continuous, in vivo molecular monitoring (e.g., of drugs or metabolites) [107]. Their signal arises from a binding-induced change in the electron transfer rate of a redox reporter. Square Wave Voltammetry (SWV) is often the preferred interrogation method.
- Validation Focus: Precision (signal stability, repeatability) and ruggedness (performance in complex matrices).
- Experimental Detail: A study comparing SWV, Differential Pulse Voltammetry (DPV), and Alternating Current Voltammetry (ACV) for interrogating a vancomycin EAB sensor found that SWV provided excellent signal-to-noise, high gain (e.g., +63.6% signal change at 300 Hz), and, crucially, supported highly accurate drift correction when the sensor was deployed in undiluted, 37 °C whole blood [107]. This demonstrates the ruggedness of the SWV-based method under physiologically relevant and challenging conditions.
- Precision Metric: The standard deviation of the signal gain for independently fabricated sensors was low (e.g., ±1.5%), indicating good manufacturing and measurement repeatability [107].
Potentiometry: Solid-Contact Ion-Selective Electrodes (SC-ISEs) for Biomedical Analysis
SC-ISEs represent a major advancement, eliminating the inner filling solution of traditional ISEs and enabling miniaturization and wearable sensors [7].
- Validation Focus: Accuracy and precision in complex biological fluids.
- Experimental Detail: A novel planar potentiometric sensor using two membranes demonstrated enhanced analytical properties. For a K+-selective version, the linear range was validated from 10⁻⁶ to 10⁻¹ M, wider than a conventional coated-disc electrode [110]. The response time was shorter, and potential drift was reduced. These improvements directly enhance the method's precision and long-term accuracy.
- Application: Such sensors are used for therapeutic drug monitoring (TDM) of pharmaceuticals with a narrow therapeutic index and for determining electrolyte levels (e.g., Na+, K+) in biological fluids, where accuracy is critical for patient diagnosis and treatment [7].
The rigorous validation of analytical methods is a non-negotiable requirement in regulatory contexts. Parameters of precision, accuracy, and ruggedness provide the framework to demonstrate that a method—whether based on the current measurement of voltammetry or the potential measurement of potentiometry—is reliable and fit for purpose. As electrochemical technologies evolve, with innovations such as in vivo EAB sensors and wearable potentiometric patches, the fundamental principles of validation remain constant. However, the specific experimental protocols must adapt to the unique challenges posed by these new applications, such as operation in complex, undiluted biological matrices. A thorough understanding and meticulous application of these validation parameters ensure the generation of high-quality, trustworthy data from the research laboratory to the clinic.
Electrochemical analysis techniques are fundamental pillars in modern analytical chemistry, playing a critical role in fields ranging from drug development to environmental monitoring. The selection of an appropriate electrochemical method is paramount for obtaining accurate, reliable, and meaningful data. This decision is fundamentally framed within the core distinction of the underlying measurement: the study of current under controlled potential in voltammetry versus the measurement of potential at zero current in potentiometry.
Voltammetry is an active technique where an applied potential forces a change in the concentration of an electroactive species at the electrode surface, and the resulting current is measured as a function of the applied potential [65]. This current is proportional to the analyte's concentration and provides rich information about reaction kinetics and mechanisms.
In contrast, Potentiometry is a passive technique that involves measuring the potential difference between a working electrode and a reference electrode under conditions of zero or negligible current flow [65] [29]. This measured potential is related to the analyte's activity (and thus concentration) via the Nernst equation.
This guide provides a structured framework to help researchers and scientists select the most appropriate technique based on their specific analytical goals, experimental constraints, and the nature of the target analyte.
Core Principles and Measurement Objectives
The essential difference in what is measured—current or potential—dictates the specific analytical information each technique can deliver. The table below summarizes the core characteristics and primary objectives of each method.
Table 1: Core Principles and Measurement Objectives of Voltammetry and Potentiometry
| Feature | Voltammetry | Potentiometry |
|---|---|---|
| Measured Quantity | Current (i) | Potential (E) |
| Applied Stimulus | Varied potential (E) | Zero current (i ≈ 0) |
| Control Variable | Potential | Current |
| Key Relationship | Current vs. Potential (i-E curve) | Potential vs. Concentration (Nernst equation) |
| Primary Analytical Information | Redox potentials, reaction kinetics, diffusion coefficients, mechanism analysis | Ion activity/concentration, equilibrium potentials, titration end-points |
| Technique Classification | Active | Passive |
The Voltammetric Approach: Interrogating Redox Behavior
Voltammetric techniques are characterized by applying a potential to a working electrode and monitoring the current generated from the reduction or oxidation of an electroactive species [65]. The resulting voltammogram provides a fingerprint of the analyte's redox behavior. Key objectives achievable with voltammetry include:
- Characterization of redox potentials for a species.
- Determination of electron transfer kinetics and reaction mechanisms [65] [29].
- Quantitative determination of organic and inorganic compounds, often at very low concentrations (sub-parts-per-billion levels for metals) [65].
- Study of adsorption processes on electrode surfaces [65].
The Potentiometric Approach: Sensing Equilibrium Potentials
Potentiometry measures the potential of an electrochemical cell under static, zero-current conditions to prevent changes in the composition of the solution being analyzed [65]. The measured potential is directly related to the activity of the target ion. Its primary objectives are:
- Direct determination of ion activity/concentration (e.g., pH, Na+, K+, Cl-) using ion-selective electrodes (ISEs) [29].
- Detection of end-points in titrations (neutralization, redox, precipitation, complex formation), where an abrupt change in potential marks the equivalence point [65].
- Monitoring reaction equilibria and thermodynamic properties in solution.
Technical Comparison and Selection Framework
Choosing between voltammetry and potentiometry requires a systematic evaluation of the analytical problem. The following table provides a direct comparison across critical parameters to guide this decision.
Table 2: Analytical Selection Framework: Voltammetry vs. Potentiometry
| Selection Criterion | Voltammetry | Potentiometry |
|---|---|---|
| Target Analyte | Electroactive species (can be oxidized or reduced) | Primarily ions (e.g., H+, Na+, K+, Ca²⁺, F⁻) |
| Primary Output | Redox properties, kinetics, concentration | Ion activity/concentration, titration end-point |
| Sensitivity | Excellent (10-12 to 10-1 M) [65] | Good for direct measurement; excellent in titration |
| Selectivity | Moderate (based on redox potential); can be enhanced with modified electrodes | High (dictated by ion-selective membrane) |
| Speed of Analysis | Rapid (seconds to minutes) | Fast for direct measurement; slower for titration |
| Sample Throughput | High | Moderate to High |
| Equipment Complexity | Moderate to High | Low to Moderate |
| Skill Requirement | Moderate to High | Low to Moderate |
| Key Strengths | Rich mechanistic information, wide linear range, trace analysis | Simplicity, cost-effectiveness, excellent ion selectivity, suitable for continuous monitoring |
| Key Limitations | Requires electroactive analyte, susceptible to fouling | Generally limited to ions, membrane stability and lifetime |
Decision Pathway
The following workflow diagram synthesizes the criteria from the tables above into a logical selection pathway for researchers.
Experimental Protocols and Methodologies
Voltammetry: Cyclic Voltammetry for Redox Characterization
Cyclic Voltammetry (CV) is a powerful and widely used technique for probing the redox behavior of electroactive species [29].
Detailed Protocol:
- Cell Assembly: Use a standard three-electrode cell configuration. The working electrode (e.g., glassy carbon, platinum, or gold disk) provides the interface for the redox reaction. The reference electrode (e.g., Ag/AgCl) maintains a constant, known potential. The counter electrode (e.g., platinum wire) completes the electrical circuit [65].
- Solution Preparation: Prepare a solution containing the analyte of interest in a suitable solvent. Add a high concentration of supporting electrolyte (e.g., 0.1 M KCl or TBAPF6) to minimize solution resistance and control the ionic strength. Deoxygenate the solution by purging with an inert gas (e.g., N2 or Ar) for 10-15 minutes to remove dissolved oxygen, which can interfere with the measurement.
- Instrumental Parameters:
- Set the initial potential.
- Set the vertex potential (the point where the scan direction reverses).
- Set the final potential (typically the same as the initial potential).
- Select a scan rate (e.g., 50-500 mV/s). Higher scan rates provide kinetic information.
- Data Acquisition: Initiate the potential sweep. The instrument applies the potential waveform and measures the resulting faradaic current. The output is a plot of current (i) vs. applied potential (E), known as a cyclic voltammogram.
- Data Analysis: Identify the peak potentials (Epa for oxidation, Epc for reduction). The reversibility of the reaction can be assessed from the peak separation (ΔEp = Epa - Epc). The peak current (ip) is proportional to the analyte concentration (for a diffusion-controlled process, ip ∝ v1/2, where v is the scan rate).
Potentiometry: Direct Potentiometric Measurement with Ion-Selective Electrodes
Direct potentiometry is commonly used for the direct determination of ion concentrations, with pH measurement being the most ubiquitous example [29].
Detailed Protocol:
- Cell Assembly: Use a two-electrode cell. The indicator electrode is an Ion-Selective Electrode (ISE) whose membrane generates a potential dependent on the activity of a specific ion. The reference electrode (e.g., Ag/AgCl or calomel) provides a stable potential reference [65].
- Calibration: Prepare a series of standard solutions of the target ion with known concentrations, covering the expected range of the unknown sample. The ionic strength should be fixed using an ionic strength adjustment buffer (ISAB). Measure the potential (E) of each standard solution and plot E (mV) vs. log(activity) or log(concentration). The plot should be linear with a slope close to the Nernstian value (59.2/z mV at 25°C, where z is the ion charge).
- Sample Measurement: Measure the potential of the unknown sample under identical conditions (same temperature, same ISAB).
- Data Analysis: Determine the concentration of the unknown sample from the calibration curve using the measured potential.
The Scientist's Toolkit: Essential Research Reagents and Materials
The successful implementation of voltammetry and potentiometry relies on a set of key materials and reagents. The following table details these essential components and their functions.
Table 3: Research Reagent Solutions for Electrochemical Analysis
| Item Name | Function/Role | Common Examples & Notes |
|---|---|---|
| Working Electrodes | Provides the surface for the electrochemical reaction to be studied. | Glassy Carbon (GC): Wide potential window, general use.Platinum (Pt): Good for oxidation reactions.Gold (Au): Often used in surface-modified studies.Mercury (Hg): Excellent negative potential range, ideal for metal ion reduction [65]. |
| Reference Electrodes | Maintains a fixed, stable, and known potential against which the working electrode potential is controlled/measured. | Ag/AgCl: Common in aqueous solutions.Saturated Calomel (SCE): Historical, but less common now.Must be chosen to be compatible with the solvent system [65]. |
| Counter Electrodes | Completes the electrical circuit in a three-electrode cell, allowing current to pass. | Platinum wire or coil is most common. Must be inert to avoid unwanted reactions [65]. |
| Ion-Selective Electrodes (ISEs) | Indicator electrode in potentiometry; generates a membrane potential specific to a target ion. | pH Glass Electrode: For H⁺.Solid-State Crystals: e.g., Fluoride ISE (LaF₃ crystal).Liquid/Polymer Membrane ISEs: e.g., for Ca²⁺, K⁺, NO₃⁻ [29]. |
| Supporting Electrolyte | Carries current to minimize solution resistance; controls ionic strength; eliminates migration current. | Salts: KCl, KNO₃, TBAPF₆ (for non-aqueous solvents). High purity is essential to prevent interference. |
| Solvents | Medium for dissolving analyte and electrolyte. | Aqueous buffers: Most common for biological/pharmaceutical applications.Non-aqueous (ACN, DMF, DCM): For organic compounds or extended potential windows. |
| Ionophores | Membrane-active complexing agents in ISEs that selectively bind to a specific ion, imparting selectivity. | Valinomycin: Highly selective for K⁺.Synthetic ionophores: Designed for specific ions like Ca²⁺ or Li⁺ [29]. |
Advanced Applications and Future Outlook
Applications in Drug Development and Environmental Analysis
The distinct capabilities of voltammetry and potentiometry make them invaluable in various fields.
- Voltammetry in Drug Development: Used for the quantitative determination of pharmaceutical compounds, studying the redox properties of drug molecules, and understanding reaction mechanisms [65]. Stripping voltammetry offers ultra-trace detection of metal impurities in drug substances.
- Potentiometry in Clinical Settings: Widely employed in clinical chemistry for blood gas analysis (pH, pCO₂, pO₂) and electrolyte panels (Na+, K+, Cl-) using automated analyzers with arrays of ISEs [29].
- Environmental Applications: Voltammetry is used for the determination of metal ion concentrations in water at sub-parts-per-billion levels [65]. Potentiometry is applied in water quality monitoring for ions like fluoride, nitrate, and ammonium.
Emerging Trends and Techniques
The field of electrochemical analysis continues to evolve, with advancements enhancing the power of both techniques.
- Advanced Voltammetry: Recent progress includes the development of microelectrodes and ultramicroelectrodes for enhanced sensitivity and spatial resolution, improved electrode materials like carbon-based nanomaterials and conductive polymers, and integration with other analytical techniques such as spectroscopy for more comprehensive analysis [29].
- Advanced Potentiometry: Advancements include the development of solid-state ISEs for improved durability and stability, the incorporation of novel sensing materials like ionophores and conductive polymers to enhance selectivity and sensitivity, and miniaturization for portable potentiometric devices for field analysis [29].
- Emerging Hybrid and Complementary Techniques: Methods like Electrochemical Impedance Spectroscopy (EIS) and Scanning Electrochemical Microscopy (SECM) are gaining prominence for studying corrosion, battery performance, biosensor interfaces, and for high-resolution imaging of electrochemical activity at surfaces [29].
Conclusion
Voltammetry and potentiometry are not competing techniques but powerful, complementary partners in the pharmaceutical analyst's toolkit. Voltammetry excels in providing highly sensitive, qualitative, and quantitative data on electroactive species like specific drugs and metabolites. In contrast, potentiometry offers robust, direct measurement of ionic activities, such as critical electrolytes, with simplicity and suitability for continuous monitoring. The future of these techniques is being shaped by trends toward miniaturization, exemplified by wearable sensors for personalized medicine; advanced materials like nanomaterials and conducting polymers that enhance signal stability; and integration with AI for smarter data analysis. For researchers in drug development, a deep understanding of both methods is indispensable for advancing therapeutic drug monitoring, ensuring product quality, and pioneering new point-of-care diagnostic solutions.
References
- 1. Electrochemical Analysis Methods: Potentiometry, ... [https://www.labmanager.com/electrochemical-analysis-methods-potentiometry-voltammetry-and-more-34242]
- 2. Voltammetric techniques of analysis: the essentials [https://link.springer.com/article/10.1007/s40828-015-0016-y]
- 3. Potentiometry - an overview [https://www.sciencedirect.com/topics/medicine-and-dentistry/potentiometry]
- 4. Video: Potentiometry: Overview [https://www.jove.com/science-education/v/14551/potentiometry-overview]
- 5. Understanding Potentiometry: Principles and Applications [https://www.ai-futureschool.com/en/chemistry/understanding-potentiometry-techniques.php]
- 6. Differential Pulse Voltammetry (DPV) [https://pineresearch.com/support-article/differential-pulse-voltammetry-dpv/]
- 7. New trends in potentiometric sensors: From design to ... [https://www.sciencedirect.com/science/article/abs/pii/S0039914025001092]
- 8. 11.7: Chapter Summary and Key Terms [https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Analytical_Chemistry_2.1_(Harvey)/11%3A_Electrochemical_Methods/11.07%3A_Chapter_Summary_and_Key_Terms]
- 9. Recent Trends in Electrochemical Methods for Real-Time ... [https://www.sciencedirect.com/science/article/abs/pii/S2451910325001085]
- 10. What are some common techniques and methods used ... [https://www.palmsens.com/potentiostat/what-are-some-common-techniques-and-methods-used-with-potentiostats-in-electrochemistry/]
- 11. Voltammetric sensors modified with nanomaterials [https://link.springer.com/article/10.1007/s44373-025-00027-9]
- 12. Perspective—Advances in Voltammetric Methods for the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11024638/]
- 13. Optical Voltammetry of redox processes inside a nanohole ... [https://arxiv.org/html/2509.01815v1]
- 14. Square Wave Voltammetry for Biosensing - by Daniel Carroll [https://danielpatrickcarroll.substack.com/p/square-wave-voltammetry-for-biosensing]
- 15. Application of potential measurements and sweep ... [https://www.sciencedirect.com/science/article/abs/pii/S0013468625008321]
- 16. Electroanalytical methods [https://en.wikipedia.org/wiki/Electroanalytical_methods]
- 17. 11.2: Potentiometric Methods [https://chem.libretexts.org/Courses/BethuneCookman_University/B-CU%3A_CH-345_Quantitative_Analysis/Book%3A_Analytical_Chemistry_2.1_(Harvey)/11%3A_Electrochemical_Methods/11.02%3A_Potentiometric_Methods]
- 18. Potentiometry - an overview | ScienceDirect Topics [https://www.sciencedirect.com/topics/chemistry/potentiometry]
- 19. 2: Potentiometric Titrations (Experiment) [https://chem.libretexts.org/Courses/University_of_California_Davis/Chem_4C_Lab%3A_General_Chemistry_for_Majors/Chem_4C%3A_Laboratory_Manual/05%3A_Potentiometric_Titrations_(Experiment)]
- 20. Tracking Lead: Potentiometric Tools and Technologies for ... [https://www.mdpi.com/1420-3049/30/17/3492]
- 21. Methods to Determine End Point of Potentiometric Titration ... [https://www.pharmaguideline.com/2021/10/methods-to-determine-end-point-of-potentiometric-titration.html]
- 22. Recent Advances in Electrochemical Sensors for the ... [https://www.mdpi.com/2079-6374/15/10/676]
- 23. 11.4: Voltammetric Methods [https://chem.libretexts.org/Courses/Northeastern_University/CHEM_1000%3A_General_Chemistry/11%3A_Electrochemical_Methods/11.4%3A_Voltammetric_Methods]
- 24. Voltammetric vs. potentiometric sensing of dopamine [https://www.sciencedirect.com/science/article/abs/pii/S0925400514008624]
- 25. Recent advances in electrochemical paper-based ... [https://www.sciencedirect.com/science/article/pii/S0013468625014446]
- 26. Quantum electroanalysis in drug discovery [https://pubs.rsc.org/en/content/articlelanding/2025/cc/d5cc01925g]
- 27. ELECTROCHEMICAL-BASED detection of drugs of abuse [https://pureportal.strath.ac.uk/en/publications/electrochemical-based-detection-of-drugs-of-abuse-recent-advances/]
- 28. – Short Stories in Instrumental Voltammetry Analytical Chemistry [https://pressbooks.bccampus.ca/instanchem1/chapter/voltammeter/]
- 29. Electrochemical Analysis Techniques: Cyclic Voltammetry ... [https://www.linkedin.com/pulse/electrochemical-analysis-techniques-cyclic-bilal-ahmad-iop3f]
- 30. Cyclic Voltammetry Basic Principles, Theory & Setup | Ossila [https://www.ossila.com/pages/cyclic-voltammetry]
- 31. Three Electrode System: The Key To Electrochemical Research [https://iestbattery.com/case/introduce-the-three-electrode-system/]
- 32. Ion-selective electrode [https://en.wikipedia.org/wiki/Ion-selective_electrode]
- 33. current measurement - Why cyclic voltammetry requires three electrodes? - Electrical Engineering Stack Exchange [https://electronics.stackexchange.com/questions/189795/why-cyclic-voltammetry-requires-three-electrodes]
- 34. Ion Selective Electrode - an overview [https://www.sciencedirect.com/topics/earth-and-planetary-sciences/ion-selective-electrode]
- 35. Ion Selective Electrode Measurement - Online ISE Analysis [https://www.ysi.com/ysi-blog/water-blogged-blog/2013/09/ion-selective-electrode-measurement-fundamentals-in-online-analysis?srsltid=AfmBOoqrNskCqAvDyKGcRdt5v6XbOp0Sis4cME41ubcXLAOFOE3IkOOX]
- 36. Electroanalysis Advances in Pharmaceutical Sciences [https://www.mdpi.com/2673-4532/6/2/12]
- 37. Differential Pulse Voltammetry (DPV) [https://www.gamry.com/resources-2/gamry-blog/high-sensitivity-analysis-with-gamry/]
- 38. emphasizing green, white, and blue analytical approaches [https://www.nature.com/articles/s41598-024-69518-w]
- 39. Square Wave Voltammetric Determination of Diclofenac in ... [https://pubmed.ncbi.nlm.nih.gov/26330859/]
- 40. Determination of Zalcitabine in Medicaments by Differential ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4590814/]
- 41. Voltammetric Determination of Alkaloids and Metabolites [https://pubmed.ncbi.nlm.nih.gov/41168644/]
- 42. The Analytical Applications of Square Wave Voltammetry ... [https://benthamopenarchives.com/abstract.php?ArticleCode=TOCBMJ-3-56]
- 43. 11.2: Potentiometric Methods [https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Analytical_Chemistry_2.1_(Harvey)/11%3A_Electrochemical_Methods/11.02%3A_Potentiometric_Methods]
- 44. Understanding Ion Selective Electrodes (ISEs) and Their ... [https://sensorex.com/ion-selective-electrodes/?srsltid=AfmBOooUzX1YdPDTAG-YLT7eIo-SLaYsD_S8HzyXXpG9859uvHA-pMne]
- 45. Potentiometric titration [https://en.wikipedia.org/wiki/Potentiometric_titration]
- 46. Potentiometric Titration Principle [https://byjus.com/chemistry/potentiometric-titration/]
- 47. Ion-Selective Electrodes [https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Analytical_Sciences_Digital_Library/Courseware/Analytical_Electrochemistry%3A_Potentiometry/03_Potentiometric_Theory/03_Ion-Selective_Electrodes]
- 48. Voltammetric Ion Sensing with Ionophore-Based ... [https://www.mdpi.com/2077-0375/12/11/1048]
- 49. Methods of Thermal Analysis as Fast and Reliable Tools ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12473009/]
- 50. Voltammetric vs. potentiometric sensing of dopamine [https://www.sciencedirect.com/science/article/pii/S0925400514008624]
- 51. Inter-Laboratory Comparison of Metabolite Measurements ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6918145/]
- 52. Comparison of two metabolomics-platforms to discover ... [https://www.sciencedirect.com/science/article/pii/S0010482524014781]
- 53. A Step-by-Step Guide to Analytical Method Development ... [https://emerypharma.com/blog/a-step-by-step-guide-to-analytical-method-development-and-validation/]
- 54. Understanding Analytical Method Development and ... [https://www.upm-inc.com/understanding-analytical-method-development-and-validation]
- 55. 11: Electrochemical Methods [https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Analytical_Chemistry_2.1_(Harvey)/11%3A_Electrochemical_Methods]
- 56. 7.5: Voltammetric Methods [https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Analytical_Sciences_Digital_Library/In_Class_Activities/Electrochemical_Methods_of_Analysis/02_Text/7%3A_Electrochemical_Analytical_Methods/7.5%3A_Voltammetric_Methods]
- 57. Emerging therapeutic drug monitoring technologies [https://pmc.ncbi.nlm.nih.gov/articles/PMC10965556/]
- 58. Electrochemical nanosensors in therapeutic ... [https://www.sciencedirect.com/science/article/pii/S0013468625009119]
- 59. Recent Advancements in Wearable Hydration-Monitoring ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12205265/]
- 60. 11.4: Voltammetric and Amperometric Methods [https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Analytical_Chemistry_2.1_(Harvey)/11%3A_Electrochemical_Methods/11.04%3A_Voltammetric_and_Amperometric_Methods]
- 61. 2.2: Potentiometric Methods [https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Analytical_Chemistry_Volume_II_(Harvey)/02%3A_Electrochemical_Methods/2.02%3A_Potentiometric_Methods]
- 62. Wearable mechanical and electrochemical sensors for real ... [https://www.nature.com/articles/s43246-024-00658-2]
- 63. 3D-Printed Carbon-Based Electrochemical Energy Storage ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12654426/]
- 64. Voltammetry [https://en.wikipedia.org/wiki/Voltammetry]
- 65. Basics of Voltammetry and Potentiometry | PPT [https://www.slideshare.net/slideshow/voltammetry-and-potentiometry/26783046]
- 66. 3D-printed conductive hydrogels for flexible ... [https://www.oaepublish.com/articles/energymater.2025.92]
- 67. Recent strategies to minimise fouling in electrochemical .. ... [https://www.degruyterbrill.com/document/doi/10.1515/revac-2015-0008/html?lang=en&srsltid=AfmBOorw8mdLQGrTeJtzQL48f_Q-UAJDgfsI4pUs0mfahddgFCCBLfEr]
- 68. Recent advances in surface modification and antifouling ... [https://www.sciencedirect.com/science/article/pii/S2451910323001126]
- 69. Recent Advances in Electrochemical Sensors for the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12563010/]
- 70. Fouling and Antifouling of Ion-Selective Electrodes [https://encyclopedia.pub/entry/59175]
- 71. Mitigation Techniques of Membranes' Biofouling in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12654448/]
- 72. Electrochemical Biosensors: A Prospective Insight to ... [https://www.sciencedirect.com/science/article/abs/pii/S2451910325001358]
- 73. Backside Calibration Potentiometry: Ion Activity ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2883721/]
- 74. Electrochemical Selectivity Achieved Using a Double ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6193764/]
- 75. Simple potentiometry and cyclic voltammetry techniques for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10811525/]
- 76. Optimization of voltammetric parameters and sensitive ... [https://www.sciencedirect.com/science/article/abs/pii/S2214714425012371]
- 77. Design and Optimization of a New Voltammetric Method for ... [https://brieflands.com/journals/ijpr/articles/125107]
- 78. Recent Developments and Challenges in Solid-Contact Ion ... [https://www.mdpi.com/1424-8220/24/13/4289]
- 79. A highly stable and flexible ion-selective patch sensor for real ... [https://mnsl-journal.springeropen.com/articles/10.1186/s40486-025-00235-3]
- 80. Stability and reproducibility study for the development of ... [https://www.sciencedirect.com/science/article/pii/S2666831925001572]
- 81. Development of reusable screen-printed ion-selective ... [https://www.sciencedirect.com/science/article/pii/S2666053925000852]
- 82. Ion-selective electrode-based sensors from the macro [https://www.sciencedirect.com/science/article/pii/S2666053924000742]
- 83. Development of two ion-selective sensors for determining ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12455838/]
- 84. Potentiometric pH measurement principle [https://www.us.endress.com/en/support-overview/learning-center/pH-principle-animation-potentiometric?srsltid=AfmBOooQSZIIf2vg5ZLt802PnQsxATTUfSFWquuYL2QPif4srRTU-L4t]
- 85. Rapid Scan Cyclic Voltammetry (RSCV) investigation of ... [https://link.springer.com/article/10.1007/s44373-025-00034-w]
- 86. Target analyte assisted sensitive electrochemical detection of ... [https://pubs.rsc.org/en/content/articlehtml/2025/lf/d5lf00006h]
- 87. Green voltammetric strategy for sensitive determination of ... [https://www.sciencedirect.com/science/article/pii/S2214180425000583]
- 88. An efficient voltammetric platform integrating ZnS ... [https://www.nature.com/articles/s41598-025-17765-w]
- 89. Electrochemical and Nanomaterial‐Based Strategies for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12598812/]
- 90. Electrochemical sensors: Types, applications, and the ... [https://www.sciencedirect.com/science/article/pii/S0956566324011060]
- 91. Tracking Lead: Potentiometric Tools and Technologies for a ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12430502/]
- 92. A high-performance reagent-less sensor based on copper(II ... [https://pubs.rsc.org/en/content/articlehtml/2025/ra/d5ra00350d]
- 93. Voltammetric Determination of the Total Content ... [https://www.mdpi.com/1420-3049/30/3/751]
- 94. A potentiometric sensor for highly selective and sensitive ... [https://www.sciencedirect.com/science/article/abs/pii/S0026265X25008501]
- 95. Electrochemical Sensors - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC3566637/]
- 96. Application to the determination of fluoride in food samples [https://www.sciencedirect.com/science/article/abs/pii/S0039914013010515]
- 97. DLS analysis of turbid | Anton Paar Wiki samples [https://wiki.anton-paar.com/se-en/dls-analysis-of-turbid-samples/]
- 98. Turbidimetry and Nephelometry Measures – PhysicsOpenLab [https://physicsopenlab.org/2021/08/18/turbidimetry-and-nephelometry-measures/]
- 99. 2020t Portable Turbidity Meters - 1974-T [https://lamotte.com/2020t-i-portable-turbidity-meters]
- 100. Voltammetric and potentiometric comparison of tendencies ... [https://www.sciencedirect.com/science/article/pii/S002207287780497X]
- 101. Assay by Potentiometric Titration in Pharmaceutical ... [https://blog.metrohmusa.com/assay-potentiometric-titration-pharmaceutical-production]
- 102. Voltammetric Determination of Active Pharmaceutical ... [https://www.mdpi.com/2227-9040/10/3/95]
- 103. Potentiometric and voltammetric determination of ... [https://www.sciencedirect.com/science/article/abs/pii/S073170850300431X]
- 104. Analytical green star area (AGSA) as a new assessment tool ... [https://pubs.rsc.org/en/content/articlehtml/2025/ra/d5ra04880j]
- 105. Validation of a Novel Potentiometric Method Based on ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7361695/]
- 106. A comparative study of green solid contact ion selective ... [https://www.nature.com/articles/s41598-023-47240-3]
- 107. Comparison of voltammetric methods used in the interrogation ... [https://pubs.rsc.org/en/content/articlehtml/2024/sd/d3sd00083d]
- 108. Analytical Method Validation: Back to Basics, Part II [https://www.chromatographyonline.com/view/analytical-method-validation-back-basics-part-ii]
- 109. Comparison of the Simple Cyclic Voltammetry (CV) and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6268035/]
- 110. A New Planar Potentiometric Sensor for In Situ ... [https://www.mdpi.com/1424-8220/24/8/2492]